Early experience with resmetirom to treat metabolic dysfunction-associated steatohepatitis with fibrosis in a real-world setting
- PMID: 40178494
- PMCID: PMC11970881
- DOI: 10.1097/HC9.0000000000000670
Early experience with resmetirom to treat metabolic dysfunction-associated steatohepatitis with fibrosis in a real-world setting
Abstract
Background: Resmetirom was conditionally approved in the United States recently for treating metabolic dysfunction-associated steatohepatitis with stage 2 and 3 fibrosis. However, its availability to patients requires preauthorization by the payors and is dispensed only through selected specialty pharmacies.
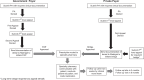
Methods: We established a multistakeholder and multistep resmetirom prescription process pivoting to a dedicated pharmacist. It incorporates liver biochemistry testing at 12 weeks and liver clinic follow-up at 6 months after starting resmetirom.
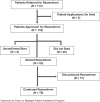
Results: Fifteen hepatology providers prescribed resmetirom to 113 patients from April 1, 2024, to November 8, 2024, with histologic eligibility in 70% and noninvasive criteria in 30%. Resmetirom treatment was approved for 110 patients (97%), including 8 patients receiving the pharmaceutical company's patient assistance and 6 patients receiving bridge support to cover the co-pay. Eighty-three patients initiated resmetirom at an average of 30 days after its prescription. Adverse events were reported by 41% of patients taking resmetirom, and they were predominantly related to gastrointestinal symptoms and pruritus and/or rash with no evidence of hypersensitivity. Thirteen patients (16%) discontinued resmetirom after an average of 25.5 days (range: 2-68 d), with 11 patients discontinuing due to adverse events. The adverse events leading to discontinuation were nausea, diarrhea, and vomiting (n=4), right upper quadrant discomfort (n=2), left lower quadrant pain (n=1), rash with pruritus (n=1), pruritus and rash with indirect hyperbilirubinemia (n=1), dizziness (n=1), and mental fogginess (n=1). Follow-up liver biochemistries available in 24 patients showed no evidence of DILI.
Conclusions: Our prescription pathway effectively dispensed resmetirom to nearly all patients who were prescribed resmetirom. One in 6 patients discontinued resmetirom, primarily due to side effects. This high discontinuation rate may be mitigated by modifying our follow-up from "prescribe and forget" to "prescribe and closely follow."
Keywords: DILI; LSM; MASH; VCTE; adverse events; fibrosis; resmetirom; side effects.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Naga Chalasani serves as a consultant to Madrigal Pharmaceuticals. He also serves as a consultant to Zydus, Ipsen, Merck, Pfizer, GSK, Akero, and Altimmune. He receives research support from Exact Sciences and Boehringer-Ingelheim. He has equity interest in Avant Sante, a contract research organization and Heligenix, a drug discovery start-up company. Niharika Samala reports a past consulting agreement with Novo Nordisk. Lindsay Yoder reports consulting agreement with Madrigal Pharmaceuticals. The remaining authors have no conflicts to report.
Figures



References
-
- Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, et al. . Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012–2024. - PubMed
-
- Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, et al. . A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390:497–509. - PubMed
-
- U.S. Food and Drug Administration. FDA approves first treatment for patients with liver scarring due to fatty liver disease. March 14, 2024. Accessed November 30, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-first-t...
-
- U.S. Food and Drug Administration. Drug Trials Snapshots: REZDIFFRA. March 14, 2024. Accessed November 20, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snaps...
-
- Matsubayashi T. Regulatory perspectives for development of drugs for treatment of Nash. U.S. Food and Drug Administration. January 29, 2021. Accessed November 30, 2024. https://www.fda.gov/drugs/news-events-human-drugs/regulatory-perspective...
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

