Zolbetuximab-related gastritis: a case report of the patient with prolonged gastrointestinal symptoms
- PMID: 40178693
- PMCID: PMC12174249
- DOI: 10.1007/s10120-025-01607-9
Zolbetuximab-related gastritis: a case report of the patient with prolonged gastrointestinal symptoms
Abstract
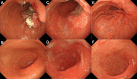
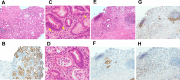
A 73-year-old male patient presented with anemia and was diagnosed with unresectable advanced gastric cancer with distant lymph node metastases. The biopsy specimen showed a poorly differentiated adenocarcinoma. Immunohistochemistry was negative for human epidermal growth factor receptor 2, positive for claudin- 18, and revealed a preserved mismatch repair status. A regimen of capecitabine, oxaliplatin, and zolbetuximab was chosen as the primary chemotherapy regimen. On day 2, the patient started complaining of nausea and decreased appetite, and his symptoms gradually worsened. Esophagogastroduodenoscopy performed on day 11 revealed an erythematous and edematous mucosa with white secretions throughout the stomach. A histopathological examination revealed epithalaxia at the surface and severe inflammatory cell infiltration in the lamina propria. These endoscopic and histological findings indicated zolbetuximab-related gastritis. His symptoms improved three weeks after the discontinuation of chemotherapy. Endoscopic and pathological improvements of the gastritis were confirmed three months after the discontinuation of zolbetuximab. This report describes the first case of prolonged severe gastrointestinal symptoms with severe gastritis caused by zolbetuximab, as demonstrated by endoscopic and histopathological evidence.
Keywords: Gastric cancer; Gastritis; Zolbetuximab.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no conflicts of interest in association with the present study. Ethics approval: Written informed consent was obtained from the patient for publication of case details and accompanying images.
Figures



References
-
- Shitara K, Lordick F, Bang Y-J, Enzinger P, Ilson D, Shah MA, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. The Lancet. 2023;401:1655–68 - PubMed
-
- Niimi T, Nagashima K, Ward JM, Minoo P, Zimonjic DB, Popescu NC, et al. claudin-18 , a Novel Downstream Target Gene for the T/EBP/NKX2.1 Homeodomain Transcription Factor, Encodes Lung- and Stomach-Specific Isoforms through Alternative Splicing. Molecular and Cellular Biology. 2001;21:7380–90 - PMC - PubMed
-
- Shimozaki K, Ooki K, Yamahata Y, Aoyama T, Yoshino K, Tamba M, et al. Managing zolbetuximab-induced nausea and vomiting: a proposal for a pragmatic approach in clinical practice. ESMO Gastrointest Oncol. 2025;7: published online
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

