Long-term left ventricular thrombosis resolution in patients receiving vitamin k antagonists: a multicenter observational study
- PMID: 40178736
- PMCID: PMC12130138
- DOI: 10.1007/s11739-025-03922-6
Long-term left ventricular thrombosis resolution in patients receiving vitamin k antagonists: a multicenter observational study
Abstract
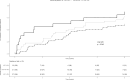
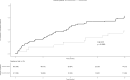
Optimal duration of anticoagulant therapy for left ventricular thrombous (LVT) is unclear. The aim of this study is to evaluate effectiveness and safety of vitamin K antagonists (VKAs) up to 12 months in patients with LVT. Patients diagnosed with LVT between 2011 and 2023 and treated with VKAs until LVT resolution or up to 12 months were enrolled in a retrospective cohort study. Primary outcome included on-treatment LVT resolution, secondary outcomes acute ischemic stroke, myocardial infarction, peripheral embolism, and major and clinically relevant non-major bleedings during the 12-month follow-up. Ninety patients were included. Median age was 66 years and 78.9% were male. Mean time in therapeutic range was 61% and 32.9% of patients received VKA monotherapy, with the remaining concomitant antiplatelet treatment. The 3, 6, 12 months cumulative incidences of LVT resolution were 27% (95% confidence intervals -95%CI-, 18%-36%), 47% (95%CI 36%-57%), and 70% (95% CI 60%-79%), respectively. At Cox regression model, reduced left ventricular ejection fraction (Hazard Ratio 0.48; 95%CI 0.24-0.95) and left-ventricular aneurysms (Hazard Ratio 0.44; 95%CI 0.22-0.88) were associated with reduced LVT resolution. One patient developed an acute ischemic stroke and one an acute myocardial infarction. Two patients developed a major and four a clinically relevant non-major bleeding. Incidence of LVT resolution appeared to be higher at 12 than at 3 and 6 months of follow-up, and the rates of on-treatment acute arterial and bleeding events were low. Reduced left ventricular ejection fraction and left-ventricular aneurysm appeared to be associated with a lower rates of LVT resolution.
Keywords: Acenocoumarol; Anticoagulants; Heparin; Venous thromboembolism; Warfarin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: E. Valeriani, G. Astorri, A. Pannunzio, D. Pastori, I.M. Palumbo, D. Menichelli, D. Santagata, L. D’Innocenzo, A. Tufano, K. Satula, R. Marcucci, A. Chistolini, F. Dragoni, T. Bucci, and P. Pignatelli have nothing to disclose. M.P. Donadini received grants or contracts from Italian Ministry of Health outside the submitted work and participation on a Data Safety Monitoring Board or Advisory Board from PlasFree outside the submitted work. E. De Candia received consulting fees and Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Viatris–Mylan and Daiichi Sanchyo outside the submitted work. M. Berteotti received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Novartis, Amgen, and Daiichi Sankyo outside the submitted work. W. Ageno received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Pfizer, Astra Zeneca, Viatris, and Sanofi outside the submitted work and participation on a Data Safety Monitoring Board or Advisory Board from Astra Zeneca, Bayer, BMS-Pfizer, Norgine, Sanofi, Viatris outside the submitted work. C. Becattini received consulting fees and Payment or honoraria for lectures presentations, speakers bureaus, manuscript writing, or educational events from Bayer Healthcare, Bristol-Myers Squibb, and Daiichi Sankyo outside the submitted work. Human and animal rights: This study was performed in accordance with the principles of Helsinki Declaration. Informed consent: All included patients consented the use of their data for study purposes.
Figures
References
-
- Levine GN, McEvoy JW, Fang JC, Ibeh C, McCarthy CP, Misra A, Shah ZI, Shenoy C, Spinler SA, Vallurupalli S, Lip GYH, Stroke N, Stroke C, American Heart Association Council on Clinical C, Council on C (2022) Management of patients at risk for and with left ventricular thrombus: a scientific statement from the American Heart Association. Circulation 146:e205–e223. 10.1161/CIR.0000000000001092 - PubMed
-
- Leow AS, Sia CH, Tan BY, Kaur R, Yeo TC, Chan MY, Tay EL, Seet RC, Loh JP, Yeo LL (2019) Characterisation of acute ischemic stroke in patients with left ventricular thrombi after myocardial infarction. J Thromb Thrombolysis 48:158–166. 10.1007/s11239-019-01829-6 - PubMed
-
- Visser CA, Kan G, Meltzer RS, Dunning AJ, Roelandt J (1985) Embolic potential of left ventricular thrombus after myocardial infarction: a two-dimensional echocardiographic study of 119 patients. J Am Coll Cardiol 5:1276–1280. 10.1016/s0735-1097(85)80336-3 - PubMed
-
- Haugland JM, Asinger RW, Mikell FL, Elsperger J, Hodges M (1984) Embolic potential of left ventricular thrombi detected by two-dimensional echocardiography. Circulation 70:588–598. 10.1161/01.cir.70.4.588 - PubMed
-
- Vaitkus PT, Barnathan ES (1993) Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 22:1004–1009. 10.1016/0735-1097(93)90409-t - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

