Gene therapy as an innovative approach to the treatment of hemophilia B-a review
- PMID: 40178764
- PMCID: PMC12367881
- DOI: 10.1007/s13353-025-00952-w
Gene therapy as an innovative approach to the treatment of hemophilia B-a review
Abstract
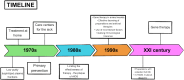
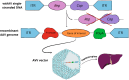
Hemophilia B is a disease that affects the human coagulation system, causing the absence or deficiency of coagulation factor IX, which may manifest itself in uncontrolled bleeding that is life-threatening to patients. Due to its inheritance, the disease more often affects men, and the severity of symptoms directly correlates with the concentration of the missing factor IX; hence, the aim of therapy is to maintain it at a level that allows for sufficient hemostasis. The basic model of treatment offered to patients is based on primary prevention with coagulation factor IX with a prolonged half-life, which, however, does not solve the numerous problems faced by patients. An innovative proposal that, despite initial concerns, is becoming more and more popular every day is the recently approved genetic therapy in Europe, which uses viral vectors to transfer the correct gene that encodes coagulation factor IX. The introduction of a recombinant gene in place of its defective counterpart seems to be a promising solution and the beginning of a new era in which genetic therapies have a chance to develop their full potential and replace existing therapeutic regimens.
Keywords: Etranacogene dezaparvovec; Gene therapy; Hemophilia B; Viral vectors.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests.
Figures
References
-
- Berntorp E, Fischer K, Hart DP et al (2021a) Haemophilia. Nat Rev Dis Primer 7(1):1–19. 10.1038/s41572-021-00285-y - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

