An unusual potassium conductance protects Caenorhabditis elegans pharyngeal muscle rhythms against environmental noise
- PMID: 40178897
- PMCID: PMC12002347
- DOI: 10.1073/pnas.2422709122
An unusual potassium conductance protects Caenorhabditis elegans pharyngeal muscle rhythms against environmental noise
Abstract
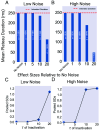
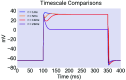
The nematode Caenorhabditis elegans feeds by rhythmic contraction and relaxation of a neuromuscular organ called the pharynx, which draws in and filters water and bacterial food. This behavior is driven by myogenic plateau potentials, long-lasting depolarizations of the pharyngeal muscle, which are timed by neuronal input from a dedicated pharyngeal nervous system. While the timing of these plateaus' initiation has received significant attention, their mechanisms of termination remain incompletely understood. In particular, it is unclear how plateaus resist early termination by hyperpolarizing current noise. Here, we present a computational model of pharyngeal plateaus against a noisy background. We propose that an unusual, rapidly inactivating potassium conductance confers exceptional noise robustness on the system. We further investigate the possibility that a similar mechanism in other systems permits switching between plateau and spiking behavior under noisy conditions.
Keywords: plateau properties; rapid K channel inactivation; synaptic noise.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures






Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Potassium-selective channelrhodopsins can exert hyper- or depolarizing effects in excitable cells of Caenorhabditis elegans, depending on experimental conditions.Genetics. 2025 Jul 9;230(3):iyaf083. doi: 10.1093/genetics/iyaf083. Genetics. 2025. PMID: 40320888
-
Pharyngeal pumping in Caenorhabditis elegans depends on tonic and phasic signaling from the nervous system.Sci Rep. 2016 Mar 15;6:22940. doi: 10.1038/srep22940. Sci Rep. 2016. PMID: 26976078 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Albertson D. G., Thompson J., The pharynx of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. Ser. B, Biol. Sci. 275, 299–325 (1976). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

