TYRA-300, an FGFR3-selective inhibitor, promotes bone growth in two FGFR3-driven models of chondrodysplasia
- PMID: 40178985
- PMCID: PMC12128972
- DOI: 10.1172/jci.insight.189307
TYRA-300, an FGFR3-selective inhibitor, promotes bone growth in two FGFR3-driven models of chondrodysplasia
Abstract
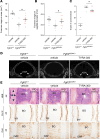
Achondroplasia (ACH) and hypochondroplasia (HCH), the two most common types of dwarfism, are each caused by FGFR3 gain-of-function mutations that result in increased FGFR3 signaling, which disrupts chondrogenesis and osteogenesis, resulting in disproportionately shortened long bones. In this study, TYRA-300, a potent and selective FGFR3 inhibitor, was evaluated in 3 genetic contexts: wild-type mice, the Fgfr3Y367C/+ mouse model of ACH, and the Fgfr3N534K/+ mouse model of HCH. In each model, TYRA-300 treatment increased nasoanal length and tibia and femur length. In the two FGFR3-altered models, TYRA-300-induced growth partially restored the disproportionality of long bones. Histologic analysis of the growth plate in Fgfr3Y367C/+ mice revealed that TYRA-300 mechanistically increased both proliferation and differentiation of chondrocytes. Importantly, children with ACH can experience medical complications due to foramen magnum stenosis, and TYRA-300 significantly improved the size and shape of the skull and foramen magnum in Fgfr3Y367C/+ mice. Spinal stenosis is also a frequent complication, and TYRA-300 increased the lumbar vertebrae length and improved the shape of the intervertebral discs in both models. Taken together, these studies demonstrate that the selective FGFR3 inhibitor TYRA-300 led to a significant increase in bone growth in two independent FGFR3-driven preclinical models as well as in wild-type mice.
Keywords: Bone biology; Bone disease; Cell biology; Drug therapy; Mouse models.
Figures




References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

