SOX9 suppresses colon cancer via inhibiting epithelial-mesenchymal transition and SOX2 induction
- PMID: 40179020
- PMCID: PMC12126244
- DOI: 10.1172/JCI184115
SOX9 suppresses colon cancer via inhibiting epithelial-mesenchymal transition and SOX2 induction
Abstract
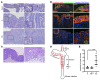
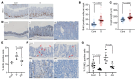
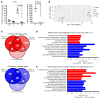
The Wnt/β-catenin pathway regulates expression of the SOX9 gene, which encodes sex-determining region Y-box (SOX) transcription factor 9, a differentiation factor and potential β-catenin regulator. Because APC tumor suppressor defects in approximately 80% of colorectal cancers (CRCs) activate the Wnt/β-catenin pathway, we studied SOX9 inactivation in CRC biology. Compared with effects of Apc inactivation in mouse colon tumors, combined Apc and Sox9 inactivation instigated more invasive tumors with epithelial-mesenchymal transition (EMT) and SOX2 stem cell factor upregulation. In an independent mouse CRC model with combined Apc, Kras, and Trp53 defects, Sox9 inactivation promoted SOX2 induction and distant metastases. About 20% of 171 human CRCs showed loss of SOX9 protein expression, which correlated with higher tumor grade. In an independent group of 376 patients with CRC, low SOX9 gene expression was linked to poor survival, earlier age at diagnosis, and increased lymph node involvement. SOX9 expression reductions in human CRC were linked to promoter methylation. EMT pathway gene expression changes were prominent in human CRCs with low SOX9 expression and in a mouse cancer model with high SOX2 expression. Our results indicate SOX9 has tumor suppressor function in CRC; its loss may promote progression, invasion, and poor prognosis by enhancing EMT and stem cell phenotypes.
Keywords: Colorectal cancer; Gastroenterology; Genetics; Mouse models; Oncology; Tumor suppressors.
Conflict of interest statement
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

