Epidemiology of Symptomatic Human Metapneumovirus Infection in the CASCADIA Community-Based Cohort - Oregon and Washington, 2022-2024
- PMID: 40179048
- PMCID: PMC11970721
- DOI: 10.15585/mmwr.mm7411a2
Epidemiology of Symptomatic Human Metapneumovirus Infection in the CASCADIA Community-Based Cohort - Oregon and Washington, 2022-2024
Abstract
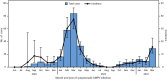
Human metapneumovirus (hMPV) is an important cause of respiratory illness. However, information about hMPV incidence, patient characteristics, and symptoms outside hospital settings is limited. During June 2022-March 2024, participants aged 6 months-49 years who were enrolled in the CASCADIA community-based cohort study submitted weekly illness surveys and nasal swabs, and completed follow-up illness surveys. Swabs collected 0-3 days before reporting new or worsening symptoms were tested for hMPV and other respiratory viruses by multiplex polymerase chain reaction. Incidence was analyzed using an exponential survival model. Among 3,549 participants, 306 had symptomatic hMPV infection, representing an average of 7.5 cases per 100 persons per year (95% CI = 6.7-8.4). Incidence was highest during January-March (adjusted hazard ratio [aHR] = 4.3; 95% CI = 3.0-6.0) compared with October-December, and among those aged 2-4 years (aHR = 5.8; 95% CI = 3.8-9.0) compared with those aged ≥40 years. The most frequently reported symptoms were cough (80.4%) and nasal congestion (71.9%). Among 252 (82.4%) participants who completed a post-illness follow-up survey, 68 (27.0%) missed work, school, or child care facility attendance. Together, these findings indicate that hMPV is a common cause of respiratory illness during late winter to spring, particularly among young children, and frequently disrupts daily activities. Understanding hMPV epidemiology can guide surveillance definitions, clinical testing, and prioritization of prevention strategies.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Ryan E. Wiegand reports ownership of common stock (with no payments received) in Sanofi and Merck. Ana A. Weil reports institutional support from Pfizer. Lea Starita reports support from Gates Ventures. Jennifer L. Kuntz reports contracts with Vir Biotechnology, Pfizer, Novartis (unrelated to the current work), and AstraZeneca. Janet A. Englund reports institutional support from AstraZeneca, GSK, Pfizer, and Moderna; receipt of consulting fees from AbbVie, AstraZeneca, GSK, Merck, Meissa Vaccines, Moderna, Pfizer, Shionogi, and Cidara Therapeutics; and receipt of honoraria from Pfizer. Helen Y. Chu reports grants or contracts from the National Institutes of Health, the Defense Advanced Research Projects Agency, Gates Ventures, Gates Foundation, Emergent Ventures, the Brotman Family, and the Alex MacMillan Foundation; receipt of lecture honoraria from the American Heart Association, the Chinese American Biomedical Society, Families of Color Seattle, the Murdoch Trust, the University of Minnesota, the University of Washington, Catholic University, Seoul, South Korea, Washington University, St. Louis, Missouri, and the American Academy of Allergy, Asthma, & Immunology (AAAAI); payment for service on advisory boards for Merck, AbbVie, Vir Biotechnology, U.S. Department of Defense, and Roche; receipt of speaker bureau payment from Medscape, Vindico Medical Education, Clinical Care Options, and Catalyst Medical Education; receipt of travel support from Medscape, Prime, Infectious Disease Society of America, the International Society of Antimicrobial Resistance, Washington University Virology Symposium, the Advisory Committee on Immunization Practices (ACIP), the Pediatric Academic Society, AAAAI, and unpaid service on ACIP. No other potential conflicts of interest were disclosed.
Figures
References
-
- Branche AR, Falsey AR. Human metapneumovirus [Chapter 159]. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandel, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Philadelphia, PA: Elsevier Saunders; 2020:2104–9.
-
- Babu TM, Feldstein LR, Saydah S, et al. CASCADIA: a prospective community-based study protocol for assessing SARS-CoV-2 vaccine effectiveness in children and adults using a remote nasal swab collection and web-based survey design. BMJ Open 2023;13:e071446. 10.1136/bmjopen-2022-071446 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

