The evolution of antibiotic resistance in Europe, 1998-2019
- PMID: 40179072
- PMCID: PMC11967945
- DOI: 10.1371/journal.ppat.1012945
The evolution of antibiotic resistance in Europe, 1998-2019
Abstract
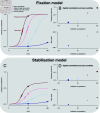
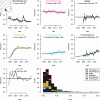
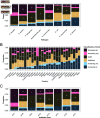
The evolutionary dynamics of antibiotic resistance are not well understood, particularly the long-term trajectories of resistance frequencies and their dependence on antibiotic consumption. Here, we systematically analyse resistance trajectories for 887 bug-drug-country combinations in Europe across 1998-2019, for eight bacterial species with a considerable resistance-associated public health burden. Our analyses support a model in which, after an initial increase, resistance frequencies reach a stable intermediate equilibrium. The plurality (37%) of analysed trajectories were best described as 'stable' (neither increasing nor decreasing). 21% of trajectories were best described as 'stabilising' - i.e. showing a transition from increasing frequency to a stable plateau; 21% as decreasing and 20% as increasing. The antibiotic consumption in a country predicts both the equilibrium frequency of the corresponding resistance and the speed at which this equilibrium is reached. Moreover, we find weak evidence that temporal fluctuations in resistance frequency are driven by temporal fluctuations in hospital antibiotic consumption. A large fraction of the variability in the speed of increase and the equilibrium level of resistance remains unexplained by antibiotic use, suggesting other factors may also drive resistance dynamics. Overall, our results indicate that ever increasing antibiotic resistance frequencies are not inevitable.
Copyright: © 2025 Emons et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Siira L, Rantala M, Jalava J, Hakanen AJ, Huovinen P, Kaijalainen T, et al. Temporal trends of antimicrobial resistance and clonality of invasive Streptococcus pneumoniae isolates in Finland, 2002 to 2006. Antimicrob Agents Chemother 2009;53(5):2066–73. doi: 10.1128/AAC.01464-08 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

