Taletrectinib in ROS1+ Non-Small Cell Lung Cancer: TRUST
- PMID: 40179330
- PMCID: PMC12118623
- DOI: 10.1200/JCO-25-00275
Taletrectinib in ROS1+ Non-Small Cell Lung Cancer: TRUST
Abstract
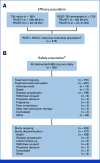
Purpose: Taletrectinib is an oral, potent, CNS-active, selective, next-generation ROS1 tyrosine kinase inhibitor (TKI). We report integrated efficacy and safety from registrational taletrectinib studies in ROS1+ non-small cell lung cancer.
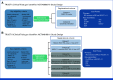
Methods: TRUST-I and TRUST-II were phase II, single-arm, open-label, nonrandomized, multicenter trials. Efficacy outcomes were pooled from TRUST-I and TRUST-II pivotal cohorts. The safety population comprised all patients treated with once-daily oral taletrectinib 600 mg pooled across the taletrectinib clinical program. The primary end point was independent review committee-assessed confirmed objective response rate (cORR). Secondary outcomes included intracranial (IC)-ORR, progression-free survival (PFS), duration of response (DOR), and safety.
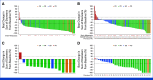
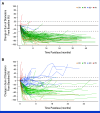
Results: As of June 7, 2024, the efficacy-evaluable population included 273 patients in TRUST-I and TRUST-II. Among TKI-naïve patients (n = 160), the cORR was 88.8% and the IC-cORR was 76.5%; in TKI-pretreated patients (n = 113), the cORR was 55.8% and the IC-cORR was 65.6%. In TKI-naïve patients, the median DOR and median PFS were 44.2 and 45.6 months, respectively. In TKI-pretreated patients, the median DOR and median PFS were 16.6 and 9.7 months. The cORR in patients with G2032R mutation was 61.5% (8 of 13). Among 352 patients treated with taletrectinib 600 mg once daily, the most frequent treatment-emergent adverse events (TEAEs) were GI events (88%) and elevated AST (72%) and ALT (68%); most were grade 1. Neurologic TEAEs were infrequent (dizziness, 21%; dysgeusia, 15%) and mostly grade 1. TEAEs leading to discontinuations (6.5%) were low.
Conclusion: Taletrectinib showed a high response rate with durable responses, robust IC activity, prolonged PFS, favorable safety, and low rates of neurologic adverse events in TKI-naïve and pretreated patients.
Trial registration: ClinicalTrials.gov NCT04919811 NCT04395677.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. - PubMed
-
- Pietrantonio F, Di Nicolantonio F, Schrock AB, et al. ALK, ROS1, and NTRK rearrangements in metastatic colorectal cancer J Natl Cancer Inst 1091-102017 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

