Maintenance therapy with romidepsin after autologous stem-cell transplant for peripheral T-cell lymphoma
- PMID: 40179392
- PMCID: PMC12466233
- DOI: 10.1182/bloodadvances.2024014263
Maintenance therapy with romidepsin after autologous stem-cell transplant for peripheral T-cell lymphoma
Abstract
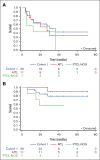
Patients with peripheral T-cell lymphoma (PTCL) have suboptimal outcomes. Autologous hematopoietic stem-cell transplantation (AHCT) is a therapeutic strategy for patients in first complete remission (CR1) or first partial remission (PR1), with median intent-to-treat progression-free survival (PFS) of 36% to 48%. Romidepsin is a histone deacetylase inhibitor used for treatment of relapsed/refractory PTCL. We present a multicenter study to evaluate the PFS of patients receiving maintenance therapy with romidepsin after AHCT. Twenty-six patients who underwent AHCT in CR1 or PR1 were evaluated for the primary end point of 2-year PFS. The exploratory cohort enrolled patients who underwent transplantation during or after CR/PR2 (n = 5) or high-risk histologies (n = 2). Patients underwent AHCT with carmustine, etoposide, cytarabine, and melphalan conditioning. Romidepsin 14 mg/m2 was started on days 42 to 80 after -AHCT every other week until 6 months of -AHCT, every 3 weeks between 6 months and 1-year of -AHCT, and every 4 weeks between 1 and 2 years of -AHCT. PFS was estimated using the Kaplan-Meier analysis. Forty-seven patients consented and 13 did not receive romidepsin. With a median progression-free follow-up of 32 months (range, 24-36), 15 of the first 25 patients in cohort 1 were progression-free after 2 years. The estimated 2-year PFS was 62% (95% confidence interval, 45-83). The most common toxicities were fatigue (n = 24, 73%), decreased platelets (n = 16, 48%), and anemia (n = 16, 48%). Although the study did not meet its desired primary efficacy end point, maintenance romidepsin was feasible, with a favorable estimated 2-year PFS of 62%. This trial was registered at www.ClinicalTrials.gov as #NCT01908777.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: N.K. reports research support from Genentech; consulting from ADC Therapeutics; and honoraria from Kyowa Kirin. S.M.H. reports research support from ADC Therapeutics, Affimed, Aileron, Celgene, CRISPR Therapeutics, Daiichi Sankyo, Forty-Seven, Inc, Kyowa Hakko Kirin, Millennium/Takeda, Seattle Genetics, Trillium Therapeutics, and Verastem/SecuraBio; and consulting from Acrotech Biopharma, ADC Therapeutics, Astex, Auxilus Pharma, Merck, C4 Therapeutics, Celgene, Cimieo Therapeutics, Daiichi Sankyo, Janssen, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Seattle Genetics, SecuraBio, Shoreline Biosciences, Inc, Takeda, Trillium Therapeutics, Tubulis, Verastem/SecuraBio, Vividion Therapeutics, and Yingli Pharma Ltd. A.J.M. reports research support from ADC Therapeutics, BeiGene, Miragen, Seattle Genetics, Merck, Bristol Myers Squibb (BMS), Incyte, and SecuraBio; and honoraria from Affimed, Imbrium Therapeutics L.P./Purdue, Janpix Ltd, Merck, Seattle Genetics, and Takeda. A.D.Z. reports financial compensation for consulting from Genentech/Roche, Gilead, Celgene, Janssen, Amgen, Novartis, Adaptive Biotechnology, MorphoSys, AbbVie, AstraZeneca, and MEI Pharma; research funding from MEI Pharmaceuticals, Genentech/Roche, BeiGene, National Institutes of Health Specialized Programs of Research Excellence principal investigator (receives salary support and funds awarded to M.S.K.); and has served on the data monitoring committee for BeiGene (Chair) and BMS/Celgene/Juno. A.N. reports research funding from Pharmacyclics/AbbVie, Kite/Gilead, and Cornerstone; consultancy for Janssen, Morphosys, Cornerstone, Epizyme, EUSA Pharma, TG Therapeutics, ADC Therapeutics, and AstraZeneca; and honoraria from Pharmacyclics/AbbVie. M.S. reports research funding from Mustang Bio, BMS, Pharmacyclics, Genentech, AbbVie, TG Therapeutics, BeiGene, AstraZeneca, Genmab, MorphoSys/Incyte, and Vincerx; and consultancy for AbbVie, Genentech, AstraZeneca, Pharmacyclics, BeiGene, BMS, MorphoSys/Incyte, Kite, Eli Lilly, Genmab, Mustang Bio, Regeneron, ADC Therapeutics, Fate Therapeutics, Janssen, and MEI Pharma. The remaining authors declare no competing financial interests.
Figures
References
-
- Ellin F, Landström J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014;124:1570–1577. - PubMed
-
- Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. - PubMed
-
- Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15:1467–1475. - PubMed
-
- Schmitz N, Trumper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–3425. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical