Platelet-activating histone/antihistone IgG complexes in anti-PF4-negative thrombosis and thrombocytopenia syndrome
- PMID: 40179418
- PMCID: PMC12402361
- DOI: 10.1182/bloodadvances.2024015076
Platelet-activating histone/antihistone IgG complexes in anti-PF4-negative thrombosis and thrombocytopenia syndrome
Abstract
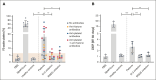
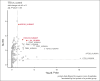
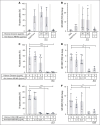
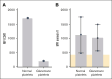
Thrombosis and thrombocytopenia syndromes (TTS) describe immune-mediated thrombotic adverse reactions after vaccination against COVID-19. Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a well-known subentity of TTS, caused by adenovirus vector-based vaccines. VITT is mediated by anti-platelet factor 4 (PF4) immunoglobulin G (IgG) antibodies, activating platelets via Fc-γ IIa receptors (FcγRIIa). We describe clinical and serological features of 18 patients with anti-PF4/heparin enzyme-linked immunosorbent assay (ELISA)-negative TTS in temporal relationship to messenger RNA (mRNA)-based COVID-19 vaccination. Symptoms began at a median of 7 (range 1 - 61) days after vaccination. Patients showed thrombocytopenia (platelet count 59 × 103/μL; range, 0 to 127 × 103/μL); petechiae (n = 7), venous thromboembolism (n = 11), arterial thrombosis (n = 6), disseminated intravascular coagulation (n = 1), and combined arterial and venous thromboses (n = 1). Twelve sera-induced FcγRIIa-dependent and caspase-independent procoagulant activation of platelets indicated by phosphatidylserine exposure and CD62P expression. We found histones precipitated with IgG fractions of TTS sera. Antibodies binding to histones were found in 8 of 12 platelet-activating sera. Ex vivo-generated histone/antihistone IgG complexes strongly activated platelets via FcγRIIa, whereas antihistone IgG alone did not. Platelet autoantibodies were detected in 7 of 12 sera targeting glycoprotein (GP) IIb/IIIa (n = 5), GPIb/IX (n = 5), and GPIa/IIa (n = 3). However, sera containing platelet anti-GPIIb/IIIa autoantibodies activated also platelets from a patient with Glanzmann thrombasthenia, making it unlikely that these autoantibodies are causative for platelet activation. Finally, 2 of 114 healthy vaccinees developed antihistone antibodies after mRNA-based COVID-19 vaccination. Our data indicate a new subentity of TTS associated with platelet-activating histone/antihistone IgG complexes. Further studies are warranted to characterize the biological and clinical role of post-mRNA-based vaccination antihistone antibodies. The SeCo trial was registered at www.ClinicalTrials.gov as #NCT04370119.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: T.T. reports grants from Deutsche Forschungsgemeinschaft during the conduct of the study (TH 2320/3-1); personal fees and other from Bristol Myers Squibb (BMS), Pfizer, and Chugai Pharma; personal fees from Bayer and Novartis; and travel support and honoraria from Novo Nordisk, Daichii Sankyo, and Leo Pharma, outside the submitted work. A.G. reports grants from Deutsche Forschungsgemeinschaft (GR 2232/9-1) and nonfinancial support from Aspen, Boehringer Ingelheim, Merck & Co, Inc (MSD), BMS, Paringenix, Bayer Healthcare, Gore Inc, Rovi, Sagent, and BioMarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, MSD, Macopharma, BMS, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and Gesellschaft für Thrombose und Hämostaseforschung e.V., outside the submitted work. J.A. reports research grants from Shire and CSL Behring; honoraria from Werfen, Stago, Siemens, Sysmex, Roche, Baxter, CSL Behring, and Sobi; and acts on advisory boards for Sobi and Novo Nordisk, outside the submitted work. C.F. reports research grants from Sobi and Biotest AG, and honoraria fees from Amgen, CSL Behring, Bayer, BioMarin, Daichii Sankyo, Sobi, Novartis, Novo Nordisk, Pfizer, and Takeda, outside the submitted work. The remaining authors declare no competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

