European Registry of Hereditary Pancreatic Diseases (EUROPAC): protocol for primary and secondary screening in individuals with inherited pancreatic disease syndromes for pancreatic ductal adenocarcinoma and complications of other pancreatic diseases
- PMID: 40180412
- PMCID: PMC11969613
- DOI: 10.1136/bmjopen-2025-100027
European Registry of Hereditary Pancreatic Diseases (EUROPAC): protocol for primary and secondary screening in individuals with inherited pancreatic disease syndromes for pancreatic ductal adenocarcinoma and complications of other pancreatic diseases
Abstract
Introduction: Pancreatic cancer is a devastating disease and one of the top causes of cancer death worldwide. Over 30% of cases are potentially avoidable, and while screening for this disease should be possible, the current methods, without risk stratification to detect high-risk groups, are unlikely to detect these individuals. A tailored screening pathway could be applied to individuals with a germline genetic cause of pancreatic cancer, which may account for around 10% of cases.
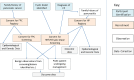
Methods and analysis: EUROPAC, although having international reach, is described here in relation to the UK only. This national prospective observational study has run for several decades but was modified into the current trial in 2019, which aims to recruit and screen 10 000 individuals with either familial pancreatic cancer or hereditary pancreatitis (HP). Applicants are assessed for eligibility by generating an individual pedigree and by attributing a family risk score (FR). Individual risk is assessed according to age. Individuals over 40 with an FR >30 are offered baseline imaging and then three yearly triplets of annual endoscopic ultrasound (EUS) and an MRI (in the third year). Those with an FR >60 are offered both EUS and MRI yearly. HP patients are screened by CT and/or MRI dependent on risk stratification using the presence of diabetes, smoking or alcohol consumption. Low-risk (absence of these factors) patients have a CT every 2 years, and high-risk (one or more of the above factors) patients have alternate yearly screening with CT, then MRI. Biospecimens are collected at pragmatic intervals with first sampling at registration to support future biomarker development to detect pancreatic cancer early. Detection of early-stage pancreatic cancer and actionable lesions will be evaluated.
Ethics and dissemination: The EUROPAC study has been reviewed and approved by the Yorkshire and Humber Research Ethics Committee (Ref 19/YH/0250). Study results will be disseminated through national and international symposium presentations and published in peer-reviewed, open-access journals. All participants provided informed consent prior to entering the study.
Trial registration number: ISRCTN62546421.
Keywords: Cancer genetics; Pancreatic disease; Pancreatic surgery; REGISTRIES.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: CH and WG are named as inventors on GB patent GBGB1806002.0; PCT/GB2019/050998, submitted by the University of Liverpool, that covers the measurement of adiponectin and IL-1Ra as a biomarker for early detection of pancreatic cancer. CH has grants from NHSE, CRUK, PCUK and LUHFT. The other authors did not declare any competing interests.
Figures
References
-
- Office for national statistics Cancer survival in England 2013 - 2017. ONS. 2019
-
- National Cancer Institute, surveillance, epidemiology, and end results program Cancer stat facts, pancreatic cancer 2014-2020. 2025
-
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–24. doi: 10.1016/S0140-6736(16)32409-6. - DOI - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous