Vascular endothelial growth factor inhibitor-induced cardiotoxicity: prospective multimodality assessment incorporating cardiovascular magnetic resonance imaging
- PMID: 40180444
- PMCID: PMC12505041
- DOI: 10.1136/heartjnl-2024-325535
Vascular endothelial growth factor inhibitor-induced cardiotoxicity: prospective multimodality assessment incorporating cardiovascular magnetic resonance imaging
Abstract
Background: Vascular endothelial growth factor inhibitors (VEGFIs) are effective anticancer agents, but are associated with cancer therapy-related cardiac dysfunction (CTRCD) and hypertension. The timing, frequency and magnitude of these toxicities are poorly defined. The objective of this study is therefore to investigate the incidence, time course and mechanisms of VEGFI-associated CTRCD and hypertension.
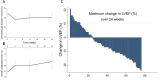
Methods: Patients commencing VEGFI underwent blood pressure (BP) monitoring, echocardiography and cardiac biomarker measurement at baseline and prospectively over 24 weeks. Serial adenosine stress perfusion cardiovascular MRI (CMR) was performed in a substudy. CTRCD was defined as left ventricular ejection fraction (LVEF) decline by ≥10 percentage points from baseline to a value <50%.
Results: 78 patients participated (68% men; age 63±11 years). 15 patients (19%) developed CTRCD, and it was evident at 4 weeks in 93% of cases. Overall, LVEF was 4.2% (95% CI: -6.2% to -2.3%, p<0.001) lower than baseline at 4 weeks. At 4 weeks, N-terminal pro-brain natriuretic peptide, but not troponin, was higher in patients with CTRCD. 62 (77%) patients developed hypertension. Home systolic and diastolic BP increased by 7.2 mm Hg (4.7-9.8, p<0.001) and 4.8 mm Hg (3.1-6.5, p<0.001), respectively, at 1 week. There was no association between change in LVEF and BP.CMR-derived LVEF, T1 relaxation times and resting myocardial blood flow (n=46) were 5.2% (-7.3% to -3.1%, p<0.001), 27 ms (-40 to -14, p<0.001) and 14.7 mL/100mL/min (-24.2 to -5.1, p=0.004), respectively, lower at 4 weeks.
Conclusion: VEGFI-associated CTRCD is frequent and occurs early. This finding has implications for prioritising early cardiac imaging follow-up after commencing treatment. Underlying mechanisms include myocardial and microvascular effects that are at least partly independent of hypertension.
Keywords: Heart Failure; Hypertension; Magnetic Resonance Imaging.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: CB is employed by the University of Glasgow which holds consultancy and research agreements for his work with Abbott Vascular, AstraZeneca, Boehringer Ingelheim, Coroventis, HeartFlow, Menarini, MSD, Novartis, Servier, Siemens Healthcare, TherOx and Valo Health. GR reports speaker honoraria from GE Healthcare and Bracco S.p.A. and a non-remunerated consultancy for Canon Medical. JE has received funding from AstraZeneca, Basilea, Bayer, Celgene, Exscientia; Exelixis; MiNa Therapeutics, Roche, Pfizer, Sierra, Lilly, Eisai, GSK, Novartis, Bicycle Therapeutics, Johnson and Johnson, CytomX, Vertex, Plexxikon, Boehringer, Athenex, Adaptimmune, Bristol Myers Squibb, MSD, Medivir, Versaterm, Nucana, Immunocore, Berg, BeiGene, Iovance, Modulate, BioLineRx, Merck Serono, Nurix Therapeutics, T3P, Janssen Clovis, Sanofi-Aventis, Halozyme, Starpharma, UCB, Sapience, Seagen, Avacta and Codiak. He has also received honoraria from Ascelia, AstraZeneca, Bayer, Bicycle Therapeutics, Bristol-Myers Squibb, Celgene, Eisai, Karus Therapeutics, Medivir, MSD, Nucana, Otsuka, Roche and Seagen. PW reports grant income from AstraZeneca, Novartis, Roche Diagnostics and Boehringer Ingelheim outside the submitted work and consultancy fees from Novo Nordisk and Benecol. BV receives research funding from BMS, Exelixis, Ipsen, MSD and Pfizer and has Honoraria from BMS, Eisai, EUSA Pharma, Ipsen, MSD and Pfizer. RJJ receives research funding from Exelixis and Roche and has honoraria from BMS, Ipsen, Novartis, Pfizer, Eisai and MSD. MCP reports research funding from Boehringer Ingelheim, Roche, SQ Innovations, AstraZeneca, Novartis, Novo Nordisk, Medtronic, Boston Scientific and Pharmacosmos; consultancy and clinical trials committees Akero, Applied Therapeutics, AnaCardio, Biosensors, Boehringer Ingelheim, Novartis, AstraZeneca, Novo Nordisk, AbbVie, Bayer, Horizon Therapeutics, Takeda, Cardiorentis, Pharmacosmos, Siemens, Lilly, Vifor, New Amsterdam, Moderna and Teikoku. NNL reports research grants from Roche Diagnostics, AstraZeneca and Boehringer Ingelheim as well as consultancy/speaker fees from Roche Diagnostics, MyoKardia, Pharmacosmos, Akero Therapeutics, CV6 Therapeutics, Jazz Pharma and Novartis all outside the submitted work. The remaining authors have nothing to disclose.
Figures




References
-
- Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43:4229–361. doi: 10.1093/eurheartj/ehac244. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
