Impact of early versus conventional kidney replacement therapy initiation in tumor lysis syndrome: a target trial emulation
- PMID: 40180676
- PMCID: PMC11968619
- DOI: 10.1186/s13613-025-01439-x
Impact of early versus conventional kidney replacement therapy initiation in tumor lysis syndrome: a target trial emulation
Abstract
Background: In the context of tumor lysis syndrome (TLS), the optimal timing and criteria for initiating kidney replacement therapy (KRT) remain unclear. This study aims to assess the effect of initiating KRT at various phosphatemia thresholds on Major Adverse Kidney Events at day 30 (MAKE30).
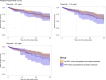
Methods and results: We retrospectively emulated a pragmatic clinical trial comparing the effect of KRT initiation at various phosphatemia thresholds versus a conventional approach during TLS on MAKE30. All consecutive patients admitted to the ICU at Saint-Louis University hospital in Paris and Angers University hospital between January 2007 and June 2020, presenting with laboratory TLS were included. The design criteria of a clinical trial were mimicked by using the cloning, censoring and weighting method. The primary outcome was the MAKE30 composite outcome, considering only KRT requirement between day 7 and day 30 for the dialysis criteria. We evaluated multiple phosphatemia thresholds to guide KRT initiation, ranging from 6.20 mg.dL-1 to 9.30 mg.dL-1. Among the initial population of 220 patients, 192 were included in the emulated trial (median age 60 years old, with non-Hodgkin Lymphoma and Acute Leukemia being the most frequent hematological malignancies). TLS-related AKI occurred in 140 patients, and 75 patients met the criteria for MAKE30. Regardless of the phosphate threshold considered, KRT initiation based on phosphate level was not associated with a significant difference in the MAKE30 rate. KRT requirement during the first 7 days (Odd Ratio [OR] 4.01 [1.65-4.86], p = 0.003) and non-renal SOFA (OR 1.39 per 1 point increment [1.25-1.57], p < 0.001) were identified as factors associated with MAKE30 (multivariable analysis).
Conclusion: Our results do not support the strategy of KRT initiation based on a sole critical phosphatemia level in TLS patients.
Keywords: Acute kidney injury; Kidney replacement therapy; Tumor lysis syndrome.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by Institutional Review Boards (IRB) (“Comité d’Evaluation de l’Ethique des projets de Recherche Biomédicale Paris Nord”—IRB 00006477—of Paris 7 University and the institutional Ethics Committee of the Angers University Hospital —2018/76). According to the French regulation, written consent was not required for this non interventional study. Patients were informed that their data might be used for research, none refused. The study was conducted following the Declaration of Helsinki principles. Competing interests: LZ reports receiving fees for lectures for MSD and Sanofi. Other authors declare no conflict of interest.
Figures

References
-
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3–11. - PubMed
-
- Darmon M, Guichard I, Vincent F, Schlemmer B, Azoulay E. Prognostic significance of acute renal injury in acute tumor lysis syndrome. Leuk Lymphoma. 2010;51:221–7. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

