Frequency of Biological Drug Use in Older Patients with Immune-Mediated Inflammatory Diseases: Results from the Large-Scale Italian VALORE Distributed Database Network
- PMID: 40180772
- PMCID: PMC12031992
- DOI: 10.1007/s40259-025-00716-2
Frequency of Biological Drug Use in Older Patients with Immune-Mediated Inflammatory Diseases: Results from the Large-Scale Italian VALORE Distributed Database Network
Abstract
Background: Limited real-world data on biological drug use in older patients with immune-mediated inflammatory diseases (IMIDs) exist despite these drugs carrying serious risks in this population.
Objective: We aimed to describe the frequency and persistence of biological drug use in older patients (≥ 65 years) with IMID, including inflammatory bowel diseases (IBDs), psoriatic arthritis/psoriasis, rheumatoid arthritis (RA), and ankylosing spondylitis, in a large Italian population.
Methods: A retrospective cohort study using the VALORE distributed claims database network from 13 Italian regions in the years 2010-2022 was performed. Older patients with IMID receiving biological drugs were included. Yearly prevalence of biological drug use and treatment persistence among incident users, from first dispensing to discontinuation/switching to another drug, was measured. Multivariable logistic regression was employed to identify treatment discontinuation predictors.
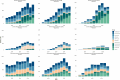
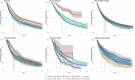
Results: The prevalence of biological drug use in older patients with IMID increased dramatically from 2010 (0.44 per 1000 residents) to 2022 (2.48 per 1000 residents). Overall, 25,284 incident users of biological drugs were identified, with a female/male ratio of 1.6 and a mean age of 71.0 (standard deviation ± 5.2) years. The median duration of follow-up was 4.2 (2.5-6.6) years, and the most common indication for use was RA (n = 8371; 33.1%). Overall, biological drug persistence was 54.4% at 1 year from treatment start. The highest persistence rates were found for vedolizumab and ustekinumab in patients with IBD (ulcerative colitis, 68.1% and 76.2%, respectively; Crohn's disease, 69.6% and 88.1%, respectively). Polypharmacy, advanced age, and female sex were identified as predictors of treatment discontinuation.
Conclusions: This study documented a significant rise in biological drug use among older patients with IMID in Italy over the last decade. Around 50% of users discontinued treatment after the first year, with even higher rates observed in very old patients with polypharmacy. These findings highlight potential concerns about the use of biological therapies in older patients and underscore the urgent need for large-scale cohort studies to address the current knowledge gaps regarding their safety and effectiveness in this vulnerable population.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement. This study was funded by the Italian Medicines Agency in the context of the multiregional pharmacovigilance project (AIFA 2012–2014: Post-marketing evaluation of the benefit–risk profile of originator biologics and biosimilars in the dermatological, rheumatological, gastroenterological and onco-hematological areas through the establishment of a single multiregional network for the integrated analysis of data from health databases, active surveillance and clinical registers—VALORE project). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Conflict of Interests: G.T. participated in advisory boards and seminars as lecturer on topics not related to the paper and was sponsored by the following pharmaceutical companies in the last 2 years: Eli Lilly; Sanofi; Amgen; Novo Nordisk; Sobi; Gilead; Celgene; Daiichi Sankyo; Takeda; and MSD. He is also scientific coordinator of the pharmacoepidemiology team at the University of Verona and of the academic spin-off “INSPIRE S.r.l.” that in the last 2 years carried out observational studies/systematic reviews on topics not related to the content of this article and which were funded by PTC Pharmaceutics, Kyowa Kirin, Shionogi, Shire, Chiesi, and Daiichi Sankyo. Y.I. is the CEO of the academic spin-off “INSPIRE S.r.l.,” which has received funding for conducting observational studies from contract research organizations (RTI Health Solutions, Pharmo Institute N.V.) and from pharmaceutical companies (Chiesi Italia, Kyowa Kirin S.r.l., Daiichi Sankyo Italia S.p.A.). Availability of Data And Material (Data Transparency): The datasets generated and/or analyzed during the current study are not publicly available because of privacy reasons. Requests to access the datasets should be directed and motivated to the corresponding author. Code Availability (Software Application or Custom Code): Not applicable. Author’s Contributions: F.S., A.S., G.C., and G.T. contributed to the writing of the article and methodology; G.P. and C.M., contributed to the conduction of the analysis; S.A.M.U and A.C. contributed to data interpretation; C.B., L.L., Y.I., O.L., M.Z., D.A., P.S., A.C., A.S., S.L., V.B., S.L., P.C., P.R., L.E., E.S., A.P., R.F.S., G.D.S., A.A., S.A.P, R.D.C., G.B., A.M.P.M, F.B., C.S., I.S., M.T., R.G., S.S.A., and M.M. contributed to data extraction and data interpretation. All the authors reviewed the final version of the article. Ethics Approvals: This study was conducted in the context of the multiregional active pharmacovigilance project called Post-marketing evaluation of the benefit–risk profile of originator biological drugs and biosimilars in the dermatological, rheumatological, gastroenterological and onco-hematological areas through the establishment of a single multiregional network for the integrated analysis of data from health databases, active surveillance and clinical registers—VALORE project, funded by the Italian Medicine Agency. The study protocol was registered in the HMA-EMA catalogue of real-world data sources and studies (EUPAS1000000211), and the ethical committees of the Academic Hospitals of Messina and Verona approved it. Consent to Participate: Not applicable. Consent to Publish: Not applicable.
Figures



References
-
- Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41:67–76. - PubMed
-
- Ingrasciotta Y, Spini A, L’Abbate L, Fiore ES, Carollo M, Ientile V, et al. Comparing clinical trial population representativeness to real-world users of 17 biologics approved for immune-mediated inflammatory diseases: an external validity analysis of 66,639 biologic users from the Italian VALORE project. Pharmacol Res. 2024;200: 107074. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

