Molecular characterization of newly diagnosed acute myeloid leukemia patients aged 60 years or older: a report from the Beat AML clinical trial
- PMID: 40180903
- PMCID: PMC11968959
- DOI: 10.1038/s41408-025-01258-0
Molecular characterization of newly diagnosed acute myeloid leukemia patients aged 60 years or older: a report from the Beat AML clinical trial
Conflict of interest statement
Competing interests: WGB has served on advisory boards of AbbVie and Syndax and has research funding from ImmuneOnc, Meryx, and Nkarta. ET has participated in advisory boards and/or consulting for Abbvie, Astellas, Daiichi-Sankyo, Servier, and Rigel. He has received research funding from Prelude Therapeutics, Schrodinger, Incyte, and Astra-Zeneca. EMS has served on the advisory boards of Astellas Pharma, AbbVie, Genentech, Daiichi Sankyo, Novartis, Amgen, Seattle Genetics, Syros Pharmaceuticals, Syndax Pharmaceuticals, Agios Pharmaceuticals, and Celgene. He is an equity holder in Auron Therapeutics. TLL has received research funding from Bio-path Holdings, Astellas Pharma, Celyad, Aptevo Therapeutics, Cleave Biosciences, Ciclomed, Jazz Pharmaceuticals, and Kura Oncology and serves on the advisory board of Servier. PAP has served on the advisory board of Agios Pharmaceuticals and is currently an employee of Servier. MRB has institutional funding from AbbVie, Ascentage, Astellas, Curadev, Gilead, Kura, Takeda. OO has served on the advisory boards of Servier, Rigel, ABBVIE, Incyte, and data safety monitoring board for Treadwell therapeutics. TK has served on advisory boards from Astellas, Rigel, Takeda; has received honoraria/consulting fees from Servier; has received institutional research funds from Abbvie, Gilead, Novartis, Syndax Pharmaceuticals. MWD is a paid consultant for Novartis, Takeda, Blueprint, Incyte, Dava Oncology, CTIBio, Syneos, Cogent, Pfizer, Dispersol. JFZ has received honoraria from advisory boards and/or consultancy from AbbVie, Daiichi Sankyo, Foghorn, Genmab, Gilead, Novartis, Sellas, Servier, Shattuck Labs, Sumitomo Dainippon, and Syndax; has received research funding from AbbVie, Arog, Astex, AstraZeneca, Gilead, Jazz, Loxo, Merck, Newave, Novartis, Sellas, Shattuck Labs, Stemline, Sumitomo Dainippon Pharma, and Zentalis. RLO has received research funds for Cellectis and consulted for Actinium, Astellas, Abbvie, Rigel, Servier. CCS has received research funds from Abbvie, BMS, Erasca, Revolution Medicines, and Zentalis and served on advisory boards for Abbvie, Genentech, and Astellas. GJS has commercial interests in BMS, Amgen, J&J. He has received fees from AbbVie, Agios, Amgen, Astellas, BMS, Incyte, Janssen, Jazz, Karyopharm, Kite, Pharmacyclics, Sanofi/Genzyme, Stemline. He has received research funds from AbbVie, Actinium, Actuate, Arog, Astellas, AltruBio, AVM Bio, BMS/ Celgene, Celator, Constellation, Daiichi-Sankyo, Deciphera, Delta-Fly, Forma, FujiFilm, Gamida, Genentech-Roche, Glycomimetics, Geron, Incyte, Karyopharm, Kiadis, Kite/Gilead, Kura, Marker, Mateon, Onconova, Pfizer, PrECOG, Regimmune, Samus, Sangamo, Sellas, Stemline, Syros, Takeda, Tolero, Trovagene, Agios, Amgen, Jazz, Orca, Ono-UK, Novartis. MS has been a consultant for Eilean Therapeutics. BJD has the following disclosures. SAB: Adela Bio, Aileron Therapeutics (inactive), Therapy Architects/ALLCRON (inactive), Cepheid, Labcorp, Nemucore Medical Innovations, Novartis, RUNX1 Research Program; SAB & Stock: Aptose Biosciences, Blueprint Medicines, Enliven Therapeutics, Iterion Therapeutics, GRAIL, Recludix Pharma; Board of Directors & Stock: Amgen, Vincerx Pharma; Board of Directors: Burroughs Wellcome Fund, CureOne (inactive); Joint Steering Committee: Beat AML LLS; Advisory Committee: Multicancer Early Detection Consortium; Founder: VB Therapeutics; Sponsored Research Agreement: Astra-Zeneca, DELiver Therapeutics, Immunoforge, Terns, Enliven Therapeutics (inactive), Recludix Pharma (inactive); Clinical Trial Funding: Novartis, Astra-Zeneca; Royalties from Patent 6958335 (Novartis exclusive license) and OHSU and Dana-Farber Cancer Institute (one Merck exclusive license, one CytoImage, Inc. exclusive license, one DELiver Therapeutics non-exclusive license, and one Sun Pharma Advanced Research Company non-exclusive license); US Patents 4326534, 6958335, 7416873, 7592142, 10473667, 10664967, 11049247. RLL is on the supervisory board of Qiagen and is a scientific advisor to Imago, Mission Bio, Syndax. Zentalis, Ajax, Bakx, Auron, Prelude, C4 Therapeutics, and Isoplexis for which he receives equity support. RLL receives research support from Ajax and Abbvie and has consulted for Incyte, Janssen, Morphosys, and Novartis. He has received honoraria from Astra Zeneca and Kura for invited lectures and from Gilead for grant reviews. UMB has been a consultant for Genentech, Daiichi Sankyo, Takeda, Pfizer, AbbVie/Genentech, and Novartis. A.S.M. has served on the advisory boards of AbbVie/Genentech, Novartis, Ryvu Therapeutics, Rigel Therapeutics, Treadwell Therapeutics, and Foghorn Therapeutics. JCB is a current equity holder in Vincerx Pharma Inc (a publicly traded company), Eilean Therapeutics, and Kurome Therapeutics. JCB holds membership on the Board of Directors or advisory committees of Vincerx, Newave, Eilean, and Kartos, and Orange Grove Bio, and holds Consultancy, Honoraria from Novartis, Trillium, Astellas, AstraZeneca, Pharmacyclics, and Syndax. YFM has received honoraria/consulting fees from BMS, Kura Oncology, BluePrint Medicines, Geron, OncLive and MD Education, VJHemOnc, and Medscape Live. YFM participated in advisory boards and received honoraria from Sierra Oncology, Stemline Therapeutics, Blueprint Medicines, Morphosys, Taiho Oncology, SOBI, Rigel Pharmaceuticals, Geron, Cogent Biosciences and Novartis. YFM received travel reimbursement from Blueprint Medicines, MD Education, and Morphosys. None of these relationships were related to this work. Ethics approval and consent to participate: This study was approved by the OSU Scientific Review Board and Western IRB centrally. All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained in accordance to the Declaration of Helsinki.
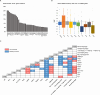
Figures
References
-
- 2016–2020 S. Cancer Stat Facts: Leukemia—Acute Myeloid Leukemia (AML) www.seer.cancer.gov. 2024.
-
- Dohner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

