Wnt signaling pathways in biology and disease: mechanisms and therapeutic advances
- PMID: 40180907
- PMCID: PMC11968978
- DOI: 10.1038/s41392-025-02142-w
Wnt signaling pathways in biology and disease: mechanisms and therapeutic advances
Abstract
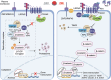
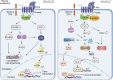
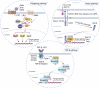
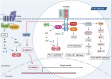
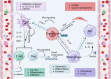
The Wnt signaling pathway is critically involved in orchestrating cellular functions such as proliferation, migration, survival, and cell fate determination during development. Given its pivotal role in cellular communication, aberrant Wnt signaling has been extensively linked to the pathogenesis of various diseases. This review offers an in-depth analysis of the Wnt pathway, detailing its signal transduction mechanisms and principal components. Furthermore, the complex network of interactions between Wnt cascades and other key signaling pathways, such as Notch, Hedgehog, TGF-β, FGF, and NF-κB, is explored. Genetic mutations affecting the Wnt pathway play a pivotal role in disease progression, with particular emphasis on Wnt signaling's involvement in cancer stem cell biology and the tumor microenvironment. Additionally, this review underscores the diverse mechanisms through which Wnt signaling contributes to diseases such as cardiovascular conditions, neurodegenerative disorders, metabolic syndromes, autoimmune diseases, and cancer. Finally, a comprehensive overview of the therapeutic progress targeting Wnt signaling was given, and the latest progress in disease treatment targeting key components of the Wnt signaling pathway was summarized in detail, including Wnt ligands/receptors, β-catenin destruction complexes, and β-catenin/TCF transcription complexes. The development of small molecule inhibitors, monoclonal antibodies, and combination therapy strategies was emphasized, while the current potential therapeutic challenges were summarized. This aims to enhance the current understanding of this key pathway.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

