Inhibiting acute, axonal DLK palmitoylation is neuroprotective and avoids deleterious effects of cell-wide DLK inhibition
- PMID: 40180913
- PMCID: PMC11968826
- DOI: 10.1038/s41467-025-58036-6
Inhibiting acute, axonal DLK palmitoylation is neuroprotective and avoids deleterious effects of cell-wide DLK inhibition
Abstract
Inhibiting dual leucine-zipper kinase (DLK) could potentially ameliorate diverse neuropathological conditions, but a direct inhibitor of DLK's kinase domain caused unintended side effects in human patients, indicative of neuronal cytoskeletal disruption. We sought a more precise intervention and show here that axon-to-soma pro-degenerative signaling requires acute, axonal palmitoylation of DLK. To identify potential modulators of this modification, we screened >28,000 compounds using a high-content imaging readout of DLK's palmitoylation-dependent subcellular localization. Several hits alter DLK localization in non-neuronal cells, reduce DLK retrograde signaling and protect cultured dorsal root ganglion neurons from neurodegeneration. Mechanistically, the two most neuroprotective compounds selectively prevent DLK's stimulus-dependent palmitoylation and subsequent recruitment to axonal vesicles, but do not affect palmitoylation of other axonal proteins assessed and avoid the cytoskeletal disruption associated with direct DLK inhibition. Our hit compounds also reduce pro-degenerative retrograde signaling in vivo, revealing a previously unrecognized neuroprotective strategy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A Patent Application No. 16/631,969 (National Stage Application of PCT/US18/42620) related to the screening method used in this manuscript was jointly filed by Temple University and Shriners Hospitals for Children. Authors S.M.H., J.N. (co-inventors) and G.M.T. (inventor) are named in the patent application. The patent application is being overseen by Temple University in accordance with its appropriate policies. The remaining authors declare no competing interests.
Figures
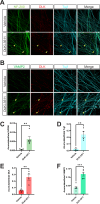





Update of
-
Novel inhibitors of acute, axonal DLK palmitoylation are neuroprotective and avoid the deleterious side effects of cell-wide DLK inhibition.bioRxiv [Preprint]. 2024 Apr 24:2024.04.19.590310. doi: 10.1101/2024.04.19.590310. bioRxiv. 2024. Update in: Nat Commun. 2025 Apr 03;16(1):3031. doi: 10.1038/s41467-025-58036-6. PMID: 38712276 Free PMC article. Updated. Preprint.
Similar articles
-
Novel inhibitors of acute, axonal DLK palmitoylation are neuroprotective and avoid the deleterious side effects of cell-wide DLK inhibition.bioRxiv [Preprint]. 2024 Apr 24:2024.04.19.590310. doi: 10.1101/2024.04.19.590310. bioRxiv. 2024. Update in: Nat Commun. 2025 Apr 03;16(1):3031. doi: 10.1038/s41467-025-58036-6. PMID: 38712276 Free PMC article. Updated. Preprint.
-
Palmitoylation controls DLK localization, interactions and activity to ensure effective axonal injury signaling.Proc Natl Acad Sci U S A. 2016 Jan 19;113(3):763-8. doi: 10.1073/pnas.1514123113. Epub 2015 Dec 30. Proc Natl Acad Sci U S A. 2016. PMID: 26719418 Free PMC article.
-
Identification of Novel Inhibitors of DLK Palmitoylation and Signaling by High Content Screening.Sci Rep. 2019 Mar 6;9(1):3632. doi: 10.1038/s41598-019-39968-8. Sci Rep. 2019. PMID: 30842471 Free PMC article.
-
An axonal stress response pathway: degenerative and regenerative signaling by DLK.Curr Opin Neurobiol. 2018 Dec;53:110-119. doi: 10.1016/j.conb.2018.07.002. Epub 2018 Jul 24. Curr Opin Neurobiol. 2018. PMID: 30053694 Free PMC article. Review.
-
Deciphering the multifunctional role of dual leucine zipper kinase (DLK) and its therapeutic potential in disease.Eur J Med Chem. 2023 Jul 5;255:115404. doi: 10.1016/j.ejmech.2023.115404. Epub 2023 Apr 20. Eur J Med Chem. 2023. PMID: 37098296 Review.
References
-
- Mata, M., Merritt, S. E., Fan, G., Yu, G. G. & Holzman, L. B. Characterization of dual leucine zipper-bearing kinase, a mixed lineage kinase present in synaptic terminals whose phosphorylation state is regulated by membrane depolarization via calcineurin. J. Biol. Chem.271, 16888–16896 (1996). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

