Data analysis strategies for the Accelerating Medicines Partnership® Schizophrenia Program
- PMID: 40180950
- PMCID: PMC11968818
- DOI: 10.1038/s41537-025-00561-w
Data analysis strategies for the Accelerating Medicines Partnership® Schizophrenia Program
Abstract
The Accelerating Medicines Partnership® Schizophrenia (AMP® SCZ) project assesses a large sample of individuals at clinical high-risk for developing psychosis (CHR) and community controls. Subjects are enrolled in 43 sites across 5 continents. The assessments include domains similar to those acquired in previous CHR studies along with novel domains that are collected longitudinally across a period of 2 years. In parallel with the data acquisition, multidisciplinary teams of experts have been working to formulate the data analysis strategy for the AMP SCZ project. Here, we describe the key principles for the data analysis. The primary AMP SCZ analysis aim is to use baseline clinical assessments and multimodal biomarkers to predict clinical endpoints of CHR individuals. These endpoints are defined for the AMP SCZ study as transition to psychosis (i.e., conversion), remission from CHR syndrome, and persistent CHR syndrome (non-conversion/non-remission) obtained at one year and two years after baseline assessment. The secondary aim is to use longitudinal clinical assessments and multimodal biomarkers from all time points to identify clinical trajectories that differentiate subgroups of CHR individuals. The design of the analysis plan is informed by reviewing legacy data and the analytic approaches from similar international CHR studies. In addition, we consider properties of the newly acquired data that are distinct from the available legacy data. Legacy data are used to assist analysis pipeline building, perform benchmark experiments, quantify clinical concepts and to make design decisions meant to overcome the challenges encountered in previous studies. We present the analytic design of the AMP SCZ project, mitigation strategies to address challenges related to the analysis plan, provide rationales for key decisions, and present examples of how the legacy data have been used to support design decisions for the analysis of the multimodal and longitudinal data. Watch Prof. Ofer Pasternak discuss his work and this article: https://vimeo.com/1023394132?share=copy#t=0 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.A. is a cofounder, serves as a member of the Board of Directors, as a scientific adviser, and holds equity in Manifest Technologies, Inc.; and is a coinventor on the following patent: Anticevic A., Murray J.D., Ji J.L.: Systems and Methods for NeuroBehavioral Relationships in Dimensional Geometric Embedding, PCT International Application No. PCT/US2119/022110, filed Mar 13, 2019. C.A. has been a consultant to or has received honoraria or grants from Acadia, Angelini, Biogen, Boehringer, Gedeon Richter, Janssen Cilag, Lundbeck, Medscape, Menarini, Minerva, Otsuka, Pfizer, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion and Takeda. D.D. has received honorary funds for one educational seminar for CSL Sequiris. G.J.P. is a full-time employee of Janssen Research & Development LLC, and a Johnson & Johnson stockholder. J.M.K. is Consultant to or receives honoraria and/or travel support and/or speakers bureau: Alkermes, Allergan, Boehringer-Ingelheim, Cerevel, Dainippon Sumitomo, H. Lundbeck, HealthRhythms, HLS Therapeutics, Indivior, Intracellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, Karuna Therapeutics/Bristol Meyer-Squibb, LB Pharmaceuticals, Mapi, Maplight, Merck, Minerva, Neurocrine, Newron, Novartis, NW PharmaTech, Otsuka, Roche, Saladax, Sunovion, Teva. J.T. is Advisor to Percison Mental Wellness. Research support from Otsuka. J.K. has received speaking or consulting fees from Janssen, Boehringer Ingelheim, ROVI and Lundbeck. P.F.P. has received research funds or personal fees from Lundbeck, Angelini, Menarini, Sunovion, Boehringer Ingelheim, Proxymm Science, Otsuka, outside the current study. Q.S.L. is an employee of Janssen Research & Development, LLC and a shareholder in Johnson & Johnson, the parent company of the Janssen companies. R.S.K. is consulting: Alkermes, Boehringer-Ingelheim. R.J.G.: Grants to Brigham and Women’s Hospital from Amgen, AstraZeneca, Kowa, and Novartis. S.W.W. has received speaking fees from the American Psychiatric Association and from Medscape Features. He has been granted US patent no. 8492418 B2 for a method of treating prodromal schizophrenia with glycine agonists. He owns stock in NW PharmaTech. S.V. is a full-time employee of Janssen Research & Development LLC, and a Johnson & Johnson stockholder. All other authors do not report any conflict of interest.
Figures


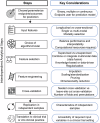

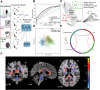

References
-
- Addington, J. et al. Clinical and functional characteristics of youth at clinical high-risk for psychosis who do not transition to psychosis. Psychol. Med.49, 1670–1677 (2019). - PubMed

