Prognostic value of circulating HPV cell-free DNA in cervical cancer using liquid biopsy
- PMID: 40180979
- PMCID: PMC11968788
- DOI: 10.1038/s41598-025-93152-9
Prognostic value of circulating HPV cell-free DNA in cervical cancer using liquid biopsy
Abstract
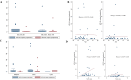
Liquid biopsies, which analyze circulating tumor cells or cell-free circulating tumor DNA (ctDNA) from blood, have emerged as promising cancer detection and monitoring tools. Specifically, human papillomavirus (HPV) cell-free (cf) DNA is gaining recognition as a prognostic marker in high-risk HPV-related cancers. However, detecting circulating markers for cervical cancer (CC) requires highly sensitive techniques to quantify circulating HPV DNA. This study aimed to evaluate the use of droplet digital PCR (ddPCR), a highly sensitive technique, for detecting and quantifying circulating HPV DNA in cervical cancer patients, both at baseline (before chemo- or radiotherapy) and during follow-up, to assess its utility as a prognostic marker. Blood samples were collected from 60 cervical cancer patients (Stages I-IV) at AIIMS, New Delhi, at baseline and three months post-treatment. Samples from 10 healthy controls were also included. Plasma was separated and stored at - 80 °C, and cfDNA was extracted from 1 ml of plasma. The presence of high-risk HPV types, HPV16 and HPV18, in cfDNA from 35 patients was assessed using ddPCR. The median concentration of cfDNA in cervical cancer patients was 9.35 ng/µL at baseline, which decreased to 7 ng/µL after three months of treatment. In healthy controls, the median cfDNA concentration was 6.95 ng/µL. ddPCR screening showed that detection rates for HPV18 and HPV16 detection were 45.71% and 82.86%, respectively. A significant correlation was observed between cf HPV16 DNA levels and tumor size, suggesting its potential as biomarker for disease burden.
Keywords: Cervical cancer; Circulating cell-free DNA; Circulating tumor DNA; Digital droplet PCR; Human papillomavirus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: Ethical approval for the current study was obtained from the AIIMS Institute ethics committee (AIIMS IEC), bearing no. IEC-208/05.05.2017. Consent for publication: All authors consent to publication.
Figures


References
-
- Taarnhøj, G. A. et al. Risk of recurrence, prognosis, and follow-up for Danish women with cervical cancer in 2005–2013: A national cohort study. Cancer124, 943–951 (2018). - PubMed
-
- Quinn, M. A. et al. Carcinoma of the cervix uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int. J. Gynaecol. Obstet.95(Suppl 1), S43-103 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

