Design, development, and preclinical evaluation of pirfenidone-loaded nanostructured lipid carriers for pulmonary delivery
- PMID: 40181013
- PMCID: PMC11968824
- DOI: 10.1038/s41598-025-90910-7
Design, development, and preclinical evaluation of pirfenidone-loaded nanostructured lipid carriers for pulmonary delivery
Abstract
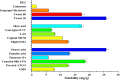
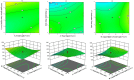
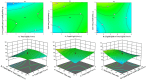
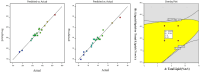
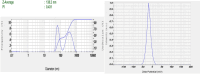
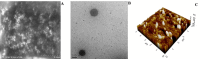
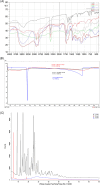
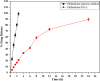
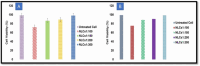
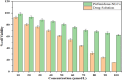
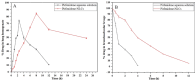
Pirfenidone is an antifibrotic and anti-inflammatory drug used for the management of idiopathic pulmonary fibrosis. The current oral delivery of PD has multiple drawbacks, including first-pass metabolism and gastrointestinal discomfort. Efforts have been made to create nanostructured lipid carriers (NLCs) using solid lipids, liquid lipids, and surfactants through an emulsification process followed by ultrasonication to achieve sustained drug release. A central composite design (CCD) utilizing response surface methods (RSMs) was employed to develop and optimize the formulation. The assessed characteristics included particle size distribution, surface topography, drug entrapment efficiency, in vitro drug release, and kinetic profiles in animal models. Cytotoxicity experiments were performed on HepG2 and Caco-2 cell lines and compared with that of PD-NLCs. The optimized formulation yielded a particle size of 159.8 ± 3.46 nm and an encapsulation efficiency of 81.4 ± 7.1% after 10 freeze-thaw cycles of homogenized lipid carriers. In vitro tests assessing various tested flow rates revealed that over 95% of the released drug was retrieved. In vitro studies showed that the PD-loaded nanostructured lipid carrier (NLC) was more cytotoxic to HepG2 and Caco-2 cells than a pure aqueous solution of the drug. Using 25% w/w sorbitol as a cryoprotectant, the findings showed no variation in the properties of NLC before and after freeze-drying. PD-NLCs carriers were shown to have better bioavailability, longer retention time in the lung, and a 15.94-targeting factor related to the PD aqueous solution. Hence, the outcomes confirmed the potential of the PD-NLCs formulation to improve the efficacy of the drug in inhalation therapy.
Keywords: Bioavailability; Caco2; Central composite design; HepG2; Lung targeting; Nanostructured lipid carriers; Pirfenidone; Pulmokinetic parameters.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Institutional Animal Ethics Committee (IAEC) of Andhra University in Visakhapatnam (IAEC No: I/IAEC/AU/013/2021WR) approved our testing technique, which was developed under the CPCSEA guidelines. The in vivo procedure was performed according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). All in vivo studies complied with the ARRIVE criteria. This study was conducted in accordance with ARRIVE guidelines. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Systematic implementation of quality-by-design (QbD) to develop NSAID-loaded nanostructured lipid carriers for ocular application: preformulation screening studies and statistical hybrid-design for optimization of variables.Drug Dev Ind Pharm. 2020 Mar;46(3):443-455. doi: 10.1080/03639045.2020.1724135. Epub 2020 Feb 9. Drug Dev Ind Pharm. 2020. PMID: 32037896
-
Formulation design, characterization, and in vitro and in vivo evaluation of nanostructured lipid carriers containing a bile salt for oral delivery of gypenosides.Int J Nanomedicine. 2019 Apr 1;14:2267-2280. doi: 10.2147/IJN.S194934. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31015758 Free PMC article.
-
Development and optimization of sulforaphane-loaded nanostructured lipid carriers by the Box-Behnken design for improved oral efficacy against cancer: in vitro, ex vivo and in vivo assessments.Artif Cells Nanomed Biotechnol. 2018;46(sup1):15-31. doi: 10.1080/21691401.2017.1408124. Epub 2017 Nov 28. Artif Cells Nanomed Biotechnol. 2018. PMID: 29183147
-
Advancements in nanotechnology for targeted drug delivery in idiopathic pulmonary fibrosis: a focus on solid lipid nanoparticles and nanostructured lipid carriers.Drug Dev Ind Pharm. 2025 Apr;51(4):285-294. doi: 10.1080/03639045.2025.2468811. Epub 2025 Feb 20. Drug Dev Ind Pharm. 2025. PMID: 39963904 Review.
-
Nanostructured lipid carriers in cancer therapy: Advances in passive and active targeting strategies.Int J Pharm. 2025 Jun 10;678:125736. doi: 10.1016/j.ijpharm.2025.125736. Epub 2025 May 17. Int J Pharm. 2025. PMID: 40389069 Review.
Cited by
-
Development and evaluation of the rivaroxaban loaded nanostructured lipid carriers for improved oral bioavailability and safety.Sci Rep. 2025 Jul 2;15(1):23350. doi: 10.1038/s41598-025-06926-6. Sci Rep. 2025. PMID: 40603393 Free PMC article.
References
-
- Hilberg, O., Simonsen, U., du Bois, R. & Bendstrup, E. Pirfenidone: Significant treatment effects in idiopathic pulmonary fibrosis. Clin. Respir. J.6, 131–143 (2012). - PubMed
-
- Richeldi, L., Yasothan, U., Kirkpatrick, P. & Pirfenidone Nat. Rev. Drug Discov.10, 489–491 (2011). - PubMed
-
- Taniguchi, H. et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J.35, 821–829 (2010). - PubMed
-
- Carter, N. J. Pirfenidone Drugs71, 1721–1732 (2011). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

