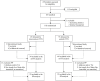
Results of a multicenter, randomized trial examining a new transition model for post-kidney transplant adolescents
- PMID: 40181098
- PMCID: PMC11968839
- DOI: 10.1038/s41598-025-95845-7
Results of a multicenter, randomized trial examining a new transition model for post-kidney transplant adolescents
Abstract
Allograft loss after pediatric kidney transplantation (KTx) is highest in adolescents and young adults. Non-adherence and Health Care Transition (HCT) are important factors, but others also contribute. In the TransNephro study patients were randomized 1:1. The intervention group was included in the Berlin Transition Program (BTP) and incorporated a central case manager, a communication app, and joined transition rounds for one year before and one year after transfer. Primary endpoint was the coefficient of variation (CoV) of the trough level of the calcineurin inhibitor as a surrogate marker for medication adherence associated with graft loss. Least square (LS) mean differences and corresponding 95% confidence intervals (CIs) were estimated using an analysis of covariance (ANCOVA) model. We assessed 220 patients for eligibility. 49 patients were randomized to the intervention group and 53 to the control group. We analyzed 84 patients in the modified intention-to-treat analysis (38 intervention, 46 controls) and 60 in the per protocol analysis (25 intervention, 35 controls). We found no difference in CoV. We saw low numbers of graft-related events and observed no differences with respect to quality of life. BTP did not improve adherence and other outcome parameters. Non-adherent patients may have decided not to participate, whilst adherence of participants was already good at study start. It is therefore achallenge to design future multicenter trials on HCT that include multiple interventions.Trial registration: ISRCTN22988897, 24/04/2014, https://doi.org/10.1186/ISRCTN22988897 .
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- Watson, A. R. et al. Transition from pediatric to adult renal services: a consensus statement by the international society of nephrology (ISN) and the international pediatric nephrology association (IPNA). Pediatr. Nephrol.26 (10), 1753–1757 (2011). - PubMed
-
- Foster, B. J. Heightened graft failure risk during emerging adulthood and transition to adult care. Pediatr. Nephrol.30, 567–576 (2015). - PubMed
-
- Prestidge, C., Romann, A., Djurdjev, O. & Matsuda-Abedini, M. Utility and cost of a renal transplant transition clinic. Pediatr. Nephrol.27, 295–302 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical