Modulating immune responses in alopecia: therapeutic insights and potential targets of antisense oligonucleotides
- PMID: 40181256
- PMCID: PMC11967052
- DOI: 10.1186/s12865-025-00685-9
Modulating immune responses in alopecia: therapeutic insights and potential targets of antisense oligonucleotides
Abstract
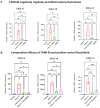
Background: Alopecia areata (AA) are hair loss disorders with distinct pathogenetic mechanisms involving immune dysregulation and microRNA modulation. AA, a T cell-mediated autoimmune disease, is characterized by sudden hair loss, with interferon-gamma (IFN-γ) playing a pivotal role in pathogenesis. The upregulation of IFN response genes, including IFN-inducible chemokines CXCL9, CXCL10, and CXCL11, in lesional skin reflects the activation of the IFN response pathway and contributes to immune cell recruitment and inflammation.
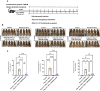
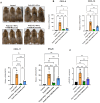
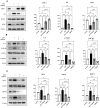
Results: Recent research highlights the role of SIRT1, a class III histone deacetylase, in modulating immune responses in AA. SIRT1 inhibition promotes the production of Th1 cytokines and chemokines, impairing inflammation, while SIRT1 activation suppresses autoreactive responses through NF-κB deacetylation and STAT3 phosphorylation. Additionally, antisense oligonucleotides (ASOs) targeting miR-485-3p show therapeutic potential in promoting hair regrowth and mitigating inflammation in murine models of androgenic alopecia (AGA) and AA.
Conclusion: Understanding chemokine dysregulation provides key insights into AA pathogenesis and highlights TAMI-M as a potential therapy for reducing inflammation and promoting hair regeneration. These findings advance the exploration of immune, microRNA, and SIRT1 pathways as targets for novel hair loss treatments.
Keywords: Alopecia areata; Antisense oligonucleotides; C3H/HeJ mice; Outer root sheath; SIRT1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The animal experimentation conducted in this study adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee (IACUC) of Biorchestra (permit numbers: BOIACUC-20230531-0002, BOIACUC-20220908-0001). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, et al. Alopecia Areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1–12. - PubMed
-
- Rathnayake D, Sinclair R. Male androgenetic alopecia. Expert Opin Pharmacother. 2010;11:1295–304. - PubMed
-
- Yip L, Sinclair RD. Antiandrogen therapy for androgenetic alopecia. Expert Rev Dermatol. 2006;1:261–9.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

