The structural and organizational aspects of human papillomavirus vaccine affecting immunization coverage in Europe: a systematic review
- PMID: 40181331
- PMCID: PMC11966883
- DOI: 10.1186/s12889-025-22343-w
The structural and organizational aspects of human papillomavirus vaccine affecting immunization coverage in Europe: a systematic review
Abstract
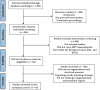
The introduction of HPV vaccinations, that can prevent most prevalent HPV-related cancers of various body districts, is a public health milestone. Despite broad immunization programs, European Health Systems face structural and organizational difficulties that hinder care. This study examined structural and organizational elements that may affect HPV vaccine coverage. We searched numerous databases from January 1, 1995 to May 15, 2023, for literature on HPV immunization research methodologies. Structural and Organizational aspects that cause HPV vaccine concerns in women and men were examined in the outcome evaluations and the research examined vaccination willingness factors. Ottawa, JBI's critical appraisal tool, and Amstar quality assessment assessed bias. A total of 10 articles from 312 studies met the inclusion criteria. Studies were undertaken in Italy, Belgium, England, Switzerland, France, the UK, and Spain. There were also combined-diverse studies in 15 and 27 European countries. Several primary healthcare strategies have increased HPV vaccination rates. These include vaccine procurement and cost-effectiveness, school-based immunization programs, electronic health databases, health professional training, health education and communication, and monitoring and surveillance.
Keywords: Cancer; Europe; Human papillomavirus; Sexually transmitted infection; Vaccination programs; Vaccine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not Applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
A systematic literature review of human papillomavirus vaccination strategies in delivery systems within national and regional immunization programs.Hum Vaccin Immunother. 2024 Dec 31;20(1):2319426. doi: 10.1080/21645515.2024.2319426. Epub 2024 Feb 27. Hum Vaccin Immunother. 2024. PMID: 38410931 Free PMC article.
-
A cost-effectiveness analysis of adult human papillomavirus vaccination strategies in Italy.Hum Vaccin Immunother. 2025 Dec;21(1):2474891. doi: 10.1080/21645515.2025.2474891. Epub 2025 Mar 17. Hum Vaccin Immunother. 2025. PMID: 40094352 Free PMC article.
-
Community-based household assessment of human papillomavirus (HPV) vaccination coverage and acceptability - HPV vaccine demonstration program, Cambodia - 2017.Vaccine. 2019 Feb 21;37(9):1202-1208. doi: 10.1016/j.vaccine.2018.12.052. Epub 2019 Jan 24. Vaccine. 2019. PMID: 30686637 Free PMC article.
-
Human papillomavirus (HPV) vaccine coverage achievements in low and middle-income countries 2007-2016.Papillomavirus Res. 2017 Dec;4:72-78. doi: 10.1016/j.pvr.2017.09.001. Epub 2017 Oct 3. Papillomavirus Res. 2017. PMID: 29179873 Free PMC article.
-
Cost-effectiveness evaluations of the 9-Valent human papillomavirus (HPV) vaccine: Evidence from a systematic review.PLoS One. 2020 Jun 2;15(6):e0233499. doi: 10.1371/journal.pone.0233499. eCollection 2020. PLoS One. 2020. PMID: 32484811 Free PMC article.
References
-
- Wang R, Pan W, Jin L, Huang W, Li Y, Wu D, et al. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Vol. 471, Cancer Letters. Elsevier Ireland Ltd; 2020. p. 88–102. - PubMed
-
- Bonanni P, Levi ,Miriam, Latham ,Nina B., Bechini ,Angela, Tiscione ,Emilia, Lai ,Piero, et al. An overview on the implementation of HPV vaccination in Europe. Human Vaccines. 2011;7(sup1):128–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous