Pharmacological effects of bile acids on polycystic ovary syndrome via the regulation of chemerin
- PMID: 40181388
- PMCID: PMC11969753
- DOI: 10.1186/s13020-025-01078-1
Pharmacological effects of bile acids on polycystic ovary syndrome via the regulation of chemerin
Abstract
Background: Polycystic ovary syndrome (PCOS) poses significant health risks for women of reproductive age, and conventional treatments typically involve anti-hormonal interventions or surgical procedures, which often lead to lifelong medication cycles and potential side effects. Bile acids have been applied in the treatment of PCOS-related conditions, including obesity and type 2 diabetes. This study aimed to investigate the effects of bile acids on a PCOS rat model and explore the underlying mechanisms involved.
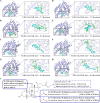
Methods: Morphological index evaluation, histopathological examination, and hormonal profiling were employed to assess the therapeutic effects of eight bile acids. A targeted proteomics was utilized to characterize and quantify highly homologous chemerin isoforms in rat serum. Network pharmacology analysis was conducted to identify potential targets and molecular mechanisms involved. Molecular docking was performed to evaluate the affinity between bile acids and farnesoid X receptor (FXR).
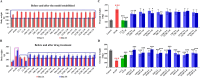
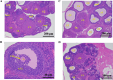
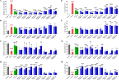
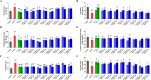
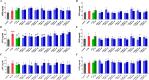
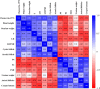
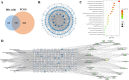
Results: Five of the eight bile acids markedly restored morphological indices, histopathological manifestations, hormonal imbalances, and chemerin isoform dysregulation. Notably, the therapeutic effects of TDCA and GUDCA on PCOS were reported for the first time. As the severity of the disease decreased, chemerin-157S was negatively correlated with progesterone (P4), estradiol (E2), antral follicles, and corpus luteum, respectively. Several chemerin-associated pathways have been identified via network pharmacology analysis. Additionally, a 7β-hydroxy group carried on the steroid skeleton of bile acids has been found to exhibit positive therapeutic efficacy in PCOS.
Conclusions: Downregulating chemerin levels via specific bile acids may be a promising therapeutic strategy for PCOS patients.
Keywords: Bile acids; Chemerin isoforms; LC/dynamic MRM-MS; Polycystic Ovarian Syndrome (PCOS); Structure–activity relationships.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures were approved by the Animal Care Committee on the Ethics of Animal Experiments of the Institute of Analysis, Guangdong Academy of Sciences (NACC) with the licence of SYXK (Guangdong) 2019-0201 for the care and use of animals. Consent for publication: All the authors have agreed to publish this work in Chinese Medicine. Competing interests: The authors declare that they have no competing interests.
Figures


Similar articles
-
Exploring the therapeutic potential of marjoram (Origanum majorana L.) in polycystic ovary syndrome: insights from serum metabolomics, network pharmacology and experimental validation.BMC Complement Med Ther. 2025 Feb 21;25(1):67. doi: 10.1186/s12906-025-04774-5. BMC Complement Med Ther. 2025. PMID: 39984989 Free PMC article.
-
Serum chemerin level in women with PCOS and its relation with the risk of spontaneous abortion.Gynecol Endocrinol. 2018 Oct;34(10):864-867. doi: 10.1080/09513590.2018.1462316. Epub 2018 Apr 16. Gynecol Endocrinol. 2018. PMID: 29658354
-
Circulating apelin and chemerin levels in patients with polycystic ovary syndrome: A meta-analysis.Front Endocrinol (Lausanne). 2023 Jan 11;13:1076951. doi: 10.3389/fendo.2022.1076951. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36714603 Free PMC article.
-
Chemerin isoform analysis in human biofluids using an LC/MRM-MS-based targeted proteomics approach with stable isotope-labeled standard.Anal Chim Acta. 2020 Dec 1;1139:79-87. doi: 10.1016/j.aca.2020.08.062. Epub 2020 Sep 3. Anal Chim Acta. 2020. PMID: 33190712
-
Chemerin and Polycystic Ovary Syndrome: A Comprehensive Review of Its Role as a Biomarker and Therapeutic Target.Biomedicines. 2024 Dec 16;12(12):2859. doi: 10.3390/biomedicines12122859. Biomedicines. 2024. PMID: 39767764 Free PMC article. Review.
References
-
- Walter K. What is polycystic ovary syndrome? JAMA. 2022;327(3):294. - PubMed
-
- Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: etiology, treatment, and genetics. Metabolism. 2019;92:108–20. - PubMed
-
- Franks S. Polycystic ovary syndrome in adolescents. Int J Obes (Lond). 2008;32(7):1035–41. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

