A retrospective research of adverse event reporting system events for voxelotor based on the FAERS database
- PMID: 40181444
- PMCID: PMC11969824
- DOI: 10.1186/s40360-025-00915-1
A retrospective research of adverse event reporting system events for voxelotor based on the FAERS database
Abstract
Background: Sickle cell disease (SCD) is a severe genetic disorder causing anemia, pain, and organ damage, affecting millions globally. Voxelotor, approved in the United States in 2019, targeted sickle cell disease pathophysiology. Despite its therapeutic benefits, concerns remain regarding its long-term safety and potential side effects, including headaches and gastrointestinal disturbances. This study used the FDA Adverse Event Reporting System (FAERS) to assess voxelotor's safety, aiming to enhance treatment strategies and clinical decision-making in SCD management.
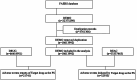
Methods: In this study, we utilized the FAERS to extract voxelotor-related adverse event reports from 2019 to 2024. We conducted descriptive and disproportionality analyses using four algorithms: Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-item Gamma Poisson Shrinkage (MGPS) to identify significant adverse event signals. The reliability of voxelotor adverse drug reactions (ADRs) was further improved by comparing with hydroxyurea ADRSs. Finally, adverse reactions were divided into acute ADRS, delayed ADRs and efficacy related reports to analyze the adverse event onset time.
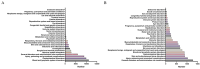
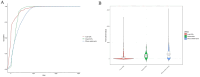
Results: A total of 16,677,340 case reports were collected in the FAERS database, of which 20,902 reports related to voxelotor were identified. Voxelotor induced adverse events occurred in 27 system organ categories (SOC). Key system organ classes affected were the blood and gastrointestinal systems. Notably, some adverse events, such as priapism and osteonecrosis, were not listed on the drug's label. The median adverse event onset time of acute ADRs, delayed ADRs and efficacy related reports were 1, 189.5 and 271 days, respectively.
Conclusion: This study systematically analyzed ADRs of voxelotor, highlighting the need for ongoing monitoring and further research on voxelotor's long-term safety and efficacy in treating sickle cell disease.
Keywords: Adverse event; Disproportionality analysis; FAERS database; Real-world data analysis; Voxelotor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The FAERS datasets are publicly accessible and anonymized, thus obviating the need for ethical approval in our current study. Consent to participate: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Real-world safety analysis of deutetrabenazine post-marketing: a disproportionality study leveraging the FDA Adverse Event Reporting System (FAERS) database.BMC Pharmacol Toxicol. 2025 Feb 21;26(1):41. doi: 10.1186/s40360-025-00872-9. BMC Pharmacol Toxicol. 2025. PMID: 39985106 Free PMC article.
-
Post-marketing safety profile of ganirelix in women: a 20-year pharmacovigilance analysis of global adverse drug event databases (2004-2024).BMC Pharmacol Toxicol. 2025 Apr 22;26(1):91. doi: 10.1186/s40360-025-00920-4. BMC Pharmacol Toxicol. 2025. PMID: 40264185 Free PMC article.
-
Safety assessment of Tafamidis: a real-world pharmacovigilance study of FDA adverse event reporting system (FAERS) events.BMC Pharmacol Toxicol. 2024 Sep 27;25(1):71. doi: 10.1186/s40360-024-00790-2. BMC Pharmacol Toxicol. 2024. PMID: 39334280 Free PMC article.
-
Romosozumab adverse event profile: a pharmacovigilance analysis based on the FDA Adverse Event Reporting System (FAERS) from 2019 to 2023.Aging Clin Exp Res. 2025 Jan 14;37(1):23. doi: 10.1007/s40520-024-02921-5. Aging Clin Exp Res. 2025. PMID: 39808360 Free PMC article.
-
Signal detection of ferric carboxymaltose-induced serious adverse events: disproportionality analysis of FAERS and VigiBase data and systematic review of case reports.Eur J Clin Pharmacol. 2025 Jul;81(7):1081-1096. doi: 10.1007/s00228-025-03849-z. Epub 2025 May 22. Eur J Clin Pharmacol. 2025. PMID: 40402208
References
-
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet (lond Engl). 2010;376:2018–31. - PubMed
-
- Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the multicenter study of hydroxyurea in sickle cell anemia. N Engl J Med. 1995;332:1317–22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

