Using patient-reported outcomes in clinical studies for cardiovascular diseases of Traditional Chinese medicine worldwide: a cross-sectional study
- PMID: 40181451
- PMCID: PMC11966848
- DOI: 10.1186/s12906-025-04864-4
Using patient-reported outcomes in clinical studies for cardiovascular diseases of Traditional Chinese medicine worldwide: a cross-sectional study
Abstract
Background and purpose: Cardiovascular diseases (CVD) represent a major global health challenge. Clinical research is increasingly leveraging patient perspectives to evaluate the efficacy of Traditional Chinese Medicine (TCM) in treating these conditions. This study reviews and summarizes the application and characteristics of Patient-reported outcomes (PROs) in TCM for CVD, the overarching goal is to provide a resource that can guide potential research directions for PROs endpoints in future TCM CVD clinical research.
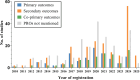
Methods: We searched clinical studies of TCM for CVD from the World Health Organization International Clinical Trials Registry Platform registered between January 1, 2010, and February 22, 2025. The outcome types, whether PRO measures (PROMs) explicitly mentioned, study design, clinical study phases,age, gender, and geographic region were analyzed. We classified the studies that explicitly specified PROs into 15 categories based on the International Classification of Diseases-11 (ICD-11),and compared their PROMs with the Core Outcome Measures in Effectiveness Trials (COMET).
Results: A total of 636 TCM CVD studies were included, of which 394 employed PROs. However, 103 studies did not specify the PROMs utilized, including 33 that involved TCM syndrome scores. None of the most frequently used PROMs were TCM-specific. The most frequently studied disease categories - chronic coronary heart diseases and heart failure - employed PROMs that were basically aligned with COMET recommendations. In contrast, other disease categories were not aligned with COMET.
Conclusion: In TCM clinical research on CVD, PROs have been commonly adopted as outcome measures, with usage steadily increasing. However, the application of TCM-specific PROMs remains limited, revealing a significant gap. PROMs recommended by COMET should be further investigated across a broader range of CVD categories. Furthermore, there is an urgent need for patient-centered research on TCM syndrome scores, highlighting the importance of developing robust, standardized TCM-specific PROMs tailored to this field.
Keywords: Cardiovascular diseases; Clinical trial registry; Patient-reported outcomes; Traditional Chinese medicine.
© 2025. The Author(s).
Conflict of interest statement
Ethics approval and consent to participate: This study used publicly available data and did not require ethical approval. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
A cross-sectional study on the application of patient-reported outcome measurements in clinical trials of traditional Chinese medicine in mainland China.Front Pharmacol. 2023 May 11;14:1159906. doi: 10.3389/fphar.2023.1159906. eCollection 2023. Front Pharmacol. 2023. PMID: 37251323 Free PMC article.
-
Developing a core outcome set on traditional Chinese medicine (COS-TCM) for chronic heart failure (CHF): a study protocol.BMJ Open. 2021 Jul 2;11(7):e047148. doi: 10.1136/bmjopen-2020-047148. BMJ Open. 2021. PMID: 34215606 Free PMC article.
-
Problems with the outcome measures in randomized controlled trials of traditional Chinese medicine in treating chronic heart failure caused by coronary heart disease: a systematic review.BMC Complement Med Ther. 2021 Aug 31;21(1):217. doi: 10.1186/s12906-021-03378-z. BMC Complement Med Ther. 2021. PMID: 34465313 Free PMC article.
-
Development of a core outcome set for myocardial infarction in clinical trials of traditional Chinese medicine: a study protocol.BMJ Open. 2019 Dec 3;9(12):e032256. doi: 10.1136/bmjopen-2019-032256. BMJ Open. 2019. PMID: 31796484 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
References
-
- Martin SS, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. 2024 heart disease and stroke statistics: a report of US and global data from the American heart association. Circulation. 2024;149:e347-913. - PubMed
-
- Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J Am Coll Cardiol. 2019;74:2529–32. - PubMed
-
- Kornowski R. Patient-reported outcome measures in cardiovascular disease. Eur Heart J Qual Care Clin Outcomes. 2023;9:119–27. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

