Multiplex Immunofluorescent Batch Labeling of Marmoset Brain Sections
- PMID: 40181645
- PMCID: PMC11968781
- DOI: 10.1002/brb3.70308
Multiplex Immunofluorescent Batch Labeling of Marmoset Brain Sections
Abstract
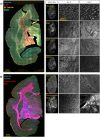
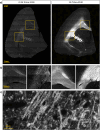
Purpose: The common marmoset is a small nonhuman primate that has emerged as a valuable animal model in neuroscience research. Accurate analysis of brain tissue is crucial to understand marmoset neurophysiology and to model neurodegenerative diseases. Many studies to date have complemented magnetic resonance imaging (MRI) with histochemical staining rather than immunofluorescent labeling, which can generate more informative and higher resolution images. There is a need for high-throughput immunolabeling and imaging methodologies to generate resources for the burgeoning marmoset field, particularly brain histology atlases to display the organization of different cell types and other structures.
Methods and findings: Here, we have characterized a set of marmoset-compatible fluorescent dyes and antibodies that label myelin, axons, dendrites, and the iron-storage protein ferritin, and developed a batch-style multiplex immunohistochemistry protocol to uniformly process large numbers of tissue slides for multiple cell-type specific markers.
Conclusion: We provide a practical guide for researchers interested in harnessing the potential of marmoset models to advance understanding of brain structure, function, and pathophysiology.
Keywords: high‐throughput; histology; immunofluorescence; marmoset; myelin.
© 2025 The Author(s). Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Atapour, N. , Majka P., Wolkowicz I. H., Malamanova D., Worthy K. H., and Rosa M. G. P.. 2019. “Neuronal Distribution Across the Cerebral Cortex of the Marmoset Monkey (Callithrix jacchus).” Cerebral Cortex (New York, N.Y.: 1991) 29, no. 9: 3836–3863. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

