Risk stratification by noninvasive tests in patients with metabolic dysfunction-associated steatotic liver disease
- PMID: 40181673
- PMCID: PMC12260630
- DOI: 10.3350/cmh.2024.1183
Risk stratification by noninvasive tests in patients with metabolic dysfunction-associated steatotic liver disease
Abstract
Background/aims: Recently, the Korean Association for the Study of the Liver (KASL) introduced a noninvasive test-based approach that uses the fibrosis-4 (FIB-4) index followed by vibration-controlled transient elastography (VCTE) to identify high-risk patients with metabolic-associated steatotic liver disease (MASLD). In this study, the KASL two-step approach was validated by assessing the risk of liver-related event (LRE) development.
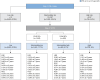
Methods: We retrospectively analyzed 8,131 patients with MASLD who underwent VCTE between 2012 and 2020. The index date was defined as the date of the VCTE measurement. Using the KASL two-step approach (FIB-4 index and subsequent VCTE), patients were stratified into four groups (low-, intermediate-low-, intermediate-high-, and high-risk groups). Outcomes, including LREs such as decompensation (DCC) or hepatocellular carcinoma (HCC) were evaluated.
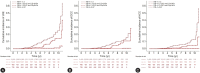
Results: During the follow-up (median 46.6 months), 86 (1.1%) patients developed LREs (39 [0.5%] with DCC and 47 [0.6%] with HCC). The KASL two-step approach classified 67.6%, 17.7%, 5.7% and 9.0% of patients in the low-, intermediate-low-, intermediate-high-, and high-risk groups, respectively. The cumulative incidences of LREs increased proportionally according to risk stratification (0.07%, 0.10%, 0.29%, and 1.51% at 3 years and 0.35%, 0.26%, 1.94% and 5.46% at 5 years). The overall accuracy in predicting LREs ranged from 67.7-99.8%. The FIB-4 index and subsequent Agile3+, Agile 4, or FibroScan aspartate aminotransferase scores showed similar predictive abilities compared to the KASL approach.
Conclusion: The KASL two-step approach is an effective and practical method for risk stratification in patients with MASLD, optimizing patient care through early identification of high-risk individuals.
Keywords: Liver-related event; Metabolic-associated steatotic liver disease; Noninvasive test; Transient elastography.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures


Comment in
-
Risk stratification of metabolic dysfunction-associated steatotic liver disease: The KASL pathway: Editorial on "Risk stratification by noninvasive tests in patients with metabolic dysfunction-associated steatotic liver disease".Clin Mol Hepatol. 2026 Jan;32(1):429-431. doi: 10.3350/cmh.2025.0420. Epub 2025 Apr 28. Clin Mol Hepatol. 2026. PMID: 40294895 Free PMC article. No abstract available.
References
-
- Kalligeros M, Vassilopoulos A, Vassilopoulos S, Victor DW, Mylonakis E, Noureddin M. Prevalence of steatotic liver disease (MASLD, MetALD, and ALD) in the United States: NHANES 2017-2020. Clin Gastroenterol Hepatol. 2024;22:1330–1332.e1334. - PubMed
-
- Miao L, Targher G, Byrne CD, Cao YY, Zheng MH. Current status and future trends of the global burden of MASLD. Trends Endocrinol Metab. 2024;35:697–707. - PubMed
-
- Perazzo H, Pacheco AG, Griep RH. Changing from NAFLD through MAFLD to MASLD: Similar prevalence and risk factors in a large Brazilian cohort. J Hepatol. 2024;80:e72–e74. - PubMed
-
- Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–1044. - PubMed
-
- Singh A, Lopez R, Lawitz E, Poordad F, Alkhouri N. Validity of non-invasive fibrosis scores to detect advanced fibrosis in patients with type 2 diabetes with suspected non-alcoholic fatty liver disease: 2017 ACG governors award for excellence in clinical research: 870. Off J Am Coll Gastroenterol | ACG. 2017;112:S491–S492.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

