Cognitive Phenotyping and Interpretation of Alzheimer Blood Biomarkers
- PMID: 40181683
- PMCID: PMC11971688
- DOI: 10.1001/jamaneurol.2025.0142
Cognitive Phenotyping and Interpretation of Alzheimer Blood Biomarkers
Abstract
Importance: Blood phosphorylated tau 217 (p-tau217) showed good performance in predicting brain amyloidosis. However, the importance of detailed cognitive phenotyping in patients without dementia when interpreting p-tau217 results remains unclear.
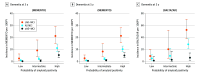
Objective: To assess whether accuracy, negative predictive value (NPV), and positive predictive value (PPV) in predicting brain amyloidosis using p-tau217 varies across clinical presentations in patients without dementia.
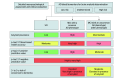
Design, setting, and participants: The study design included 2 observational, prospective cohort studies: The Cohort of Outpatients From French Research Memory Centers in Order to Improve Knowledge on Alzheimer's Disease and Related Disorders (MEMENTO), with enrollment from 2011 to 2014 and 5 years of follow-up, and the Biomarker of Amyloid Peptide and Alzheimer's Disease Risk (BALTAZAR) cohort study, with enrollment from 2010 to 2015 and 3 years of follow-up. Both are multicenter cohorts conducted in French memory clinics. Participants without dementia were included for analysis if they had baseline blood p-tau217 measurement and a known amyloid status through cerebrospinal fluid amyloid β (Aβ)-42/Aβ-40 ratio or positron emission tomography. They presented with either subjective cognitive impairment (SCI), mild cognitive impairment (MCI) with a common Alzheimer disease (AD) phenotype (cAD-MCI: amnestic syndrome of hippocampal type, posterior cortical atrophy, or logopenic primary progressive aphasia), or MCI with uncommon AD or other phenotypes (uAD-MCI). Data were analyzed from May to September 2024.
Exposures: Blood p-tau217 concentrations.
Main outcomes and measures: Brain amyloidosis probabilities were derived from p-tau217 logistic regressions including age, gender, and APOE genotype. Published and internally developed cut points with 90% sensitivity and specificity were used.
Results: A total of 776 participants from the MEMENTO cohort (N = 2323 participants) and 193 participants from the BALTAZAR cohort (N = 1040) were included in this analysis. In the MEMENTO cohort (median [IQR] age, 71 [65-76] years; 444 female [57%]), brain amyloidosis prevalence was 16.5% (20 of 121) in SCI, 45.9% (78 of 170) in cAD-MCI, and 24.5% (119 of 485) in uAD-MCI. Area under the receiver operating characteristic curve for predicting brain amyloidosis with p-tau217 models was 0.78 (95% CI, 0.66-0.89), 0.91 (95% CI, 0.86-0.95), and 0.87 (95% CI, 0.84-0.91) in the SCI, cAD-MCI, and uAD-MCI subgroups, respectively. External cut points resulted in a PPV of 60.0%, 90.0%, and 74.5% in the SCI, cAD-MCI, and uAD-MCI subgroups, respectively. NPV ranged from 84.2% to 90.2%. With internally developed cut points, PPVs were 52.6%, 84.0%, and 72.3% in the SCI, cAD-MCI, and uAD-MCI subgroups, respectively. NPVs were high (91.7%-94.6%) in all subgroups. Rates of incident dementia strongly increased with the probability of brain amyloidosis in the cAD-MCI subgroup. Replicated analyses in the BALTAZAR cohort provided similar results.
Conclusions and relevance: Results from 2 clinical cohorts suggest that amyloid prevalence varied across cognitive phenotypes and was associated with the diagnostic performance of blood p-tau217 models to determine brain amyloidosis. Comprehensive cognitive phenotyping beyond the basic characterization of SCI, MCI, or dementia should accompany the use of blood biomarkers in clinical practice to avoid misdiagnosis due to false positives.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

