Multicentre comparison of various microaxial pump devices as a bridge to durable assist device implantation
- PMID: 40181705
- PMCID: PMC12287827
- DOI: 10.1002/ehf2.15282
Multicentre comparison of various microaxial pump devices as a bridge to durable assist device implantation
Abstract
Aims: Patients with acute decompensated advanced heart failure requiring left ventricular assist device (LVAD) implantation often experience progressive cardiac function deterioration, negatively impacting surgical outcomes. This study aimed to assess the efficacy of different microaxial flow pump (mAFP) support devices (Impella®) in achieving optimal left ventricular unloading for preconditioning and facilitating definitive treatment in this high-risk patient cohort.
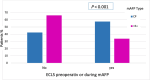
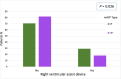
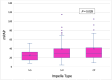
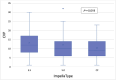
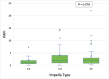
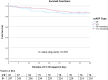
Methods and results: A retrospective analysis was conducted across 19 high-volume European centres. The study population included patients transitioning from temporary to durable circulatory support over a 7.5-year period, with a median follow-up of 1 year. Patients were categorized based on mAFP support capacity: those receiving high-flow support (>5 L/min, '5+') and those with lower-flow support (3.5 L/min, 'CP'). Patients who were initially treated with CP but subsequently upgraded to 5+ support were classified in the 5+ group. Demographic and clinical characteristics, mobilization, right heart function, and organ dysfunction outcomes were analysed. A total of 339 patients received preoperative mAFP support prior to LVAD implantation. The 5+ group comprised 247 patients (73%), including 38 patients who were upgraded from CP, while the CP group included 92 patients (27%). Baseline demographic and clinical characteristics were comparable between groups, except for mobilization status, which showed significant differences (P < 0.001). Patients in the 5+ group achieved higher rates of full and partial mobilization compared to the CP group. Extracorporeal life support (ECLS) was more frequently required in the CP group than in the 5+ group (40.5% vs. 33.8%; P < 0.001). Additionally, right ventricular assist device (RVAD) implantation was significantly more common in the CP group (29.2% vs. 18.2%; P = 0.026). Patients in the 5+ group demonstrated greater reductions in both vasoactive inotropic scores (P = 0.006) and inotropic scores (P = 0.008). Furthermore, liver dysfunction (P = 0.016), renal failure (P = 0.041), and the need for dialysis (P = 0.013) were significantly more prevalent in the CP group. There were no significant differences between the two groups in terms of LVAD operative duration (P = 0.637) or cardiopulmonary bypass time (P = 0.408).
Conclusions: High-flow mAFP devices (+5) provided superior haemodynamic support, enhanced left ventricular unloading, and reduced dependence on catecholamines compared to lower-flow CP devices. These improvements were associated with lower rates of right ventricular failure, renal dysfunction, and liver injury. However, no statistically significant difference was observed between mAFP groups regarding 30-day mortality rates.
Keywords: Cardiogenic shock; Durable mechanical circulatory support; Impella; Left heart failure; Microaxial flow pump.
© 2025 The Author(s). ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
Dr Oezkur and Dr Meyer reports research funding, travel funding, and speaker fees, from Abiomed. Dr Potapov reports consulting honoraria speaker honoraria, proctoring fees, and institutional grants from Abiomed, Abbot, Medtronic, and Recovery Therapeutics. Dr Treede reports advisory board honoraria from Abiomed. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Dr A. Bernhardt, Dr Oezkur, and Dr Meyer received speaker honoraria and travel support from Abiomed. All other authors declare no competing interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

