Adalimumab Monotherapy or Combination Therapy With Methotrexate in Paediatric Uveitis: Data From the AIDA Network Uveitis Registry
- PMID: 40181732
- PMCID: PMC12326217
- DOI: 10.1111/ceo.14534
Adalimumab Monotherapy or Combination Therapy With Methotrexate in Paediatric Uveitis: Data From the AIDA Network Uveitis Registry
Abstract
Background: The study objective was to compare the effectiveness of adalimumab (ADA) in monotherapy and in combination with methotrexate (MTX) for paediatric noninfectious uveitis (NIU).
Methods: Registry-based observational study. Children receiving ADA for active uveitis were divided into the ADA monotherapy group (group 1) and the ADA plus MTX combination group (group 2).
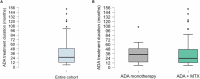
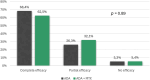
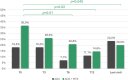
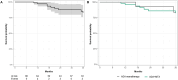
Results: Eighty four children were enrolled (146 eyes): 22 in group 1 (26.2%) and 62 in group 2 (73.8%). ADA effectiveness was complete in 48 children (57.1%), partial in 23 (27.4%) and absent in 4 (5.3%), without any differences across the groups (p = 0.89). Fewer relapses per 100 PY occurred after ADA treatment both in group 1 (280.0 vs. 23.0, p = 0.005) and in group 2 (297.9 vs. 86.0, p < 0.001). The final BCVA was similar between groups 1 and 2 [median 1.0 (IQR 0.3) and 1.0 (IQR 0.3), respectively, p = 0.55]. A statistically significant steroid-sparing effect was observed in the entire cohort and in group 2 at the 6-month (p = 0.01 and p = 0.01), 12-month (p = 0.02 and p = 0.02), and last follow-up (p = 0.045 and p = 0.045). The estimated ADA retention rate was 97.1% at 12 months, 87.7% at 24 months, and 82.6% at 36 months, without a statistically significant difference among the groups (p = 0.77).
Conclusions: ADA monotherapy could be equally effective as its combination with MTX in both preventing uveitis relapses and preserving visual acuity in paediatric NIU, with comparable retention rates over 36 months of treatment. The steroid-sparing effect of ADA monotherapy warrants further extensive evaluation to define its optimal placement in the therapeutic strategy for paediatric NIU.
Keywords: adalimumab; methotrexate; monotherapy; noninfectious uveitis; paediatric ophthalmology.
© 2025 The Author(s). Clinical & Experimental Ophthalmology published by John Wiley & Sons Australia, Ltd on behalf of Royal Australian and New Zealand College of Ophthalmologists.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ramanan A. V., Dick A. D., Benton D., et al., “A Randomised Controlled Trial of the Clinical Effectiveness, Safety and Cost‐Effectiveness of Adalimumab in Combination With Methotrexate for the Treatment of Juvenile Idiopathic Arthritis Associated Uveitis (SYCAMORE Trial),” Trials 15, no. 14 (2014): 14, 10.1186/1745-6215-15-14. - DOI - PMC - PubMed
-
- Quartier P., Baptiste A., Despert V., et al., “ADJUVITE: A Double‐Blind, Randomised, Placebo‐Controlled Trial of Adalimumab in Early Onset, Chronic, Juvenile Idiopathic Arthritis‐Associated Anterior Uveitis,” Annals of the Rheumatic Diseases 77, no. 7 (2018): 1003–1011, 10.1136/annrheumdis-2017-212089. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

