Inflammation and Immune-Based Scores Predict Prognosis for Patients with Hepatocellular Carcinoma: A Pilot Single-Center Study
- PMID: 40181742
- PMCID: PMC12001462
- DOI: 10.5152/tjg.2025.24407
Inflammation and Immune-Based Scores Predict Prognosis for Patients with Hepatocellular Carcinoma: A Pilot Single-Center Study
Abstract
Background/aims: Hepatocellular carcinoma (HCC) ranks as a major contributor to cancer-related deaths. Systemic inflammation plays a pivotal role in HCC development and progression. Thus, we aimed to determine the impact of inflammation- and immune-based scores in predicting the prognosis of HCC.
Materials and methods: In this retrospective study, patients with HCC were enrolled between 2010 and 2020. The purpose of this retrospective study was to evaluate the impact of various biomarkers, including baseline alpha-fetoprotein (AFP) levels, C-reactive protein (CRP)-albumin-lymphocyte (CALLY) index, neutrophil-to-CRP (N/CRP) ratio, systemic immune-inflammation index (SII), albumin-bilirubin (ALBI) score, neutrophil-to-lymphocyte ratio (NLR), and aspartate aminotransferase (AST)-to-alanine aminotransferase (ALT) (AST/ALT) ratio (AAR), on survival, invasion of vascular tracts, metastasis, and treatment responses.
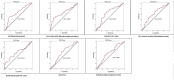
Results: A total of 199 patients with complete (n = 44) and non-complete (n = 145) treatment response groups were enrolled in the study. All scores for the non-complete response group were statistically significant (P < .05). The areas under the curves for predicting a non-complete response group were 0.651, 0.649, 0.636, 0.625, 0.613, 0.609, and 0.600 for AFP, CALLY index, AAR, SII, N/CRP ratio, ALBI score, and NLR, respectively. These results are consistent with the assessment of mortality and HCC progression.
Conclusion: Our results indicate that these biomarkers could serve as powerful prognostic tools for HCC.
Keywords: C-reactive protein–albumin–lymphocyte index; Hepatocellular carcinoma; alpha-fetoprotein; inflammation.
Conflict of interest statement
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous