Piezo1 in PASMCs: Critical for Hypoxia-Induced Pulmonary Hypertension Development
- PMID: 40181773
- PMCID: PMC12036789
- DOI: 10.1161/CIRCRESAHA.124.325475
Piezo1 in PASMCs: Critical for Hypoxia-Induced Pulmonary Hypertension Development
Abstract
Background: Pulmonary hypertension (PH) is a life-threatening and progressive yet incurable disease. The hallmarks of PH comprise (1) sustained contraction and (2) excessive proliferation of pulmonary arterial smooth muscle cells (PASMCs). A major stimulus to which PASMCs are exposed during PH development is altered mechanical stress, originating from increased blood pressure, changes in blood flow velocity, and a progressive stiffening of pulmonary arteries. Mechanosensitive ion channels, including Piezo1 (Piezo-type mechanosensitive ion channel component-1), perceive such mechanical stimuli and translate them into a variety of cellular responses, including contractility or proliferation. Thus, the objective of the present study was to elucidate the specific role of Piezo1 in PASMCs for PH development and progression.
Methods: The cell-type specific function of Piezo1 in PH was assessed in (1) PASMCs and lung tissues from patients with PH and (2) 2 mouse strains characterized by smooth muscle cell-specific, conditional Piezo1 knockout. Taking advantage of these strains, the smooth muscle cell-specific role of Piezo1 in PH development and progression was assessed in isolated, perfused, and ventilated mouse lungs, wire myography, and proliferation assays. Finally, in vivo function of smooth muscle cell-specific Piezo1 knockout was evaluated upon induction of chronic hypoxia-induced PH in these mice with insights into pulmonary vascular cell senescence.
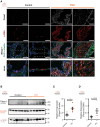
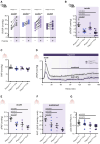
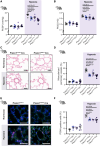
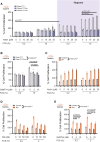
Results: Compared with healthy controls, PASMCs from patients with PH featured an elevated Piezo1 expression and increased proliferative phenotype. Smooth muscle cell-specific Piezo1 deletion, as confirmed via quantitative real-time polymerase chain reaction and patch clamp recordings, prevented the hypoxia-induced increase in PASMC proliferation in mice. Moreover, Piezo1 knockout reduced hypoxic pulmonary vasoconstriction in isolated, perfused, and ventilated mouse lungs, endothelial-denuded pulmonary arteries, and hemodynamic measurements in vivo. Consequently, Piezo1-deficient mice were considerably protected against chronic hypoxia-induced PH development with ameliorated right heart hypertrophy and improved hemodynamic function. In addition, distal pulmonary capillaries were preserved in the Piezo1-knockout mice, associated with a lower number of senescent endothelial cells.
Conclusions: This study provides evidence that Piezo1 expressed in PASMCs is critically involved in the pathogenesis of PH by controlling pulmonary vascular tone, arterial remodeling, and associated lung capillary rarefaction due to endothelial cell senescence.
Keywords: blood pressure; cellular senescence; endothelial cells; hypertension, pulmonary; hypoxia; vascular remodeling.
Conflict of interest statement
None.
Figures



References
-
- Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:1527–1538. doi: 10.1016/j.jacc.2008.01.024 - PubMed
-
- Veith C, Varturk-Ozcan I, Wujak M, Hadzic S, Wu CY, Knoepp F, Kraut S, Petrovic A, Gredic M, Pak O, et al. . SPARC, a novel regulator of vascular cell function in pulmonary hypertension. Circulation. 2022;145:916–933. doi: 10.1161/CIRCULATIONAHA.121.057001 - PubMed
-
- Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano-Subias P, Ferrari P, et al. ; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

