Nodule organogenesis in Medicago truncatula requires local stage-specific auxin biosynthesis and transport
- PMID: 40181792
- PMCID: PMC12002018
- DOI: 10.1093/plphys/kiaf133
Nodule organogenesis in Medicago truncatula requires local stage-specific auxin biosynthesis and transport
Abstract
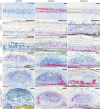
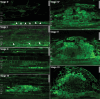
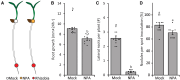
The importance of auxin in plant organ development, including root nodule formation, is well known. The spatiotemporal distribution pattern of auxin during nodule development has been illustrated using auxin reporter constructs. However, our understanding of how this pattern is established and maintained remains elusive. Here, we studied how the auxin gradient is associated with the spatiotemporal expression patterns of known auxin biosynthesis and transport genes at different stages of nodule development in Medicago (Medicago truncatula). In addition, we examined the Medicago PIN-FORMED10 (MtPIN10) expression pattern and polar positioning on the cell membrane during nodule primordium development to investigate auxin flux. RNA interference and the application of auxin biosynthesis inhibitors were used to demonstrate the importance of auxin biosynthesis and transport at the initial stages of nodulation. Our results show that upon rhizobium inoculation before the first cell divisions, a specific subset of Medicago YUCCA (MtYUC) and MtPIN genes, as well as Medicago LIKE AUXIN RESISTANT2 (MtLAX2), are expressed in the pericycle and contribute to the creation of an auxin maximum. Overall, we demonstrate that the dynamic spatiotemporal expression of both MtYUC and MtPIN genes results in specific auxin outputs during the different stages of nodule primordia and nodule meristem formation.
© The Author(s) 2025. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures





Similar articles
-
MtLAX2, a Functional Homologue of the Arabidopsis Auxin Influx Transporter AUX1, Is Required for Nodule Organogenesis.Plant Physiol. 2017 May;174(1):326-338. doi: 10.1104/pp.16.01473. Epub 2017 Mar 31. Plant Physiol. 2017. PMID: 28363992 Free PMC article.
-
The peptide GOLVEN10 alters root development and noduletaxis in Medicago truncatula.Plant J. 2024 May;118(3):607-625. doi: 10.1111/tpj.16626. Epub 2024 Feb 15. Plant J. 2024. PMID: 38361340
-
MtbHLH1, a bHLH transcription factor involved in Medicago truncatula nodule vascular patterning and nodule to plant metabolic exchanges.New Phytol. 2011 Jul;191(2):391-404. doi: 10.1111/j.1469-8137.2011.03718.x. Epub 2011 Jun 17. New Phytol. 2011. PMID: 21679315 Free PMC article.
-
Rhizobial infection is associated with the development of peripheral vasculature in nodules of Medicago truncatula.Plant Physiol. 2013 May;162(1):107-15. doi: 10.1104/pp.113.215111. Epub 2013 Mar 27. Plant Physiol. 2013. PMID: 23535942 Free PMC article.
-
From Inception to Maturation: Recent Insights in Nodule Organogenesis.Physiol Plant. 2025 May-Jun;177(3):e70277. doi: 10.1111/ppl.70277. Physiol Plant. 2025. PMID: 40401688 Review.
Cited by
-
Auxin! here you go again: Spatiotemporal dynamic regulation of auxin promotes proper nodule formation in Medicago truncatula.Plant Physiol. 2025 Apr 30;198(1):kiaf177. doi: 10.1093/plphys/kiaf177. Plant Physiol. 2025. PMID: 40315303 Free PMC article. No abstract available.
References
-
- Allen EK, Allen ON, Newman AS. Pseudonodulation of leguminous plants induced by 2-bromo-3,5- dichlorobenzoic acid. Am J Bot. 1953:40(6):429–435. 10.1002/j.1537-2197.1953.tb06502.x - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

