Hexokinase2-engineered T cells display increased anti-tumor function
- PMID: 40181966
- PMCID: PMC11965122
- DOI: 10.3389/fimmu.2025.1477929
Hexokinase2-engineered T cells display increased anti-tumor function
Abstract
Background: T cells face significant metabolic challenges in the tumor microenvironment (TME), where cancer cells monopolize critical nutrients like glucose and amino acids. This metabolic competition supports tumor growth while impairing T-cell anti-tumor responses, partly by reducing glycolytic function. Hexokinase 2 (HK2), a key enzyme in glycolysis, plays a pivotal role in maintaining T-cell functionality.
Methods: To enhance T-cell function, primary human T cells were genetically engineered to overexpress HK2 alongside a tumor-specific receptor. These engineered T cells were tested in vitro and in vivo to evaluate their metabolic and therapeutic efficacy.
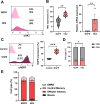
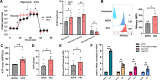
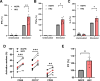
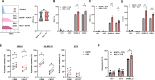
Results: HK2-engineered T cells exhibited increased glycolytic capacity, leading to enhanced cytokine secretion, activation marker expression, and metabolic activity compared to controls. In vivo studies using a human tumor xenograft model demonstrated the superior therapeutic efficacy of HK2-engineered T cells, including delayed tumor growth and improved survival.
Conclusion: HK2 overexpression improves T-cell metabolic fitness and functionality in hostile TMEs, offering a promising foundation for the development of next-generation immunotherapies targeting T-cell metabolism.
Keywords: T-cells; TCR; cellular immunotherapy; hexokinase 2; immunometabolism.
Copyright © 2025 Zur, Zurinam, Radman, Funaro Balouka, Borodianskiy-Shteinberg, Saur and Cohen.
Conflict of interest statement
CC and RZ are inventors on a submitted PCT Application No. PCT/IL2023/050248 related to this study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Genetically engineering glycolysis in T cells increases their antitumor function.J Immunother Cancer. 2024 Jul 4;12(7):e008434. doi: 10.1136/jitc-2023-008434. J Immunother Cancer. 2024. PMID: 38964783 Free PMC article.
-
NF-κB-inducing kinase maintains T cell metabolic fitness in antitumor immunity.Nat Immunol. 2021 Feb;22(2):193-204. doi: 10.1038/s41590-020-00829-6. Epub 2021 Jan 4. Nat Immunol. 2021. PMID: 33398181 Free PMC article.
-
Enhanced Cancer Immunotherapy by Chimeric Antigen Receptor-Modified T Cells Engineered to Secrete Checkpoint Inhibitors.Clin Cancer Res. 2017 Nov 15;23(22):6982-6992. doi: 10.1158/1078-0432.CCR-17-0867. Epub 2017 Sep 14. Clin Cancer Res. 2017. PMID: 28912137
-
Remodeling metabolic fitness: Strategies for improving the efficacy of chimeric antigen receptor T cell therapy.Cancer Lett. 2022 Mar 31;529:139-152. doi: 10.1016/j.canlet.2022.01.006. Epub 2022 Jan 7. Cancer Lett. 2022. PMID: 35007698 Review.
-
Fundamentals of T Cell Metabolism and Strategies to Enhance Cancer Immunotherapy.Front Immunol. 2021 Mar 18;12:645242. doi: 10.3389/fimmu.2021.645242. eCollection 2021. Front Immunol. 2021. PMID: 33815400 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

