Remodeling of immune system functions by extracellular vesicles
- PMID: 40181981
- PMCID: PMC11966064
- DOI: 10.3389/fimmu.2025.1549107
Remodeling of immune system functions by extracellular vesicles
Abstract
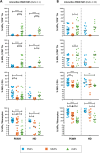
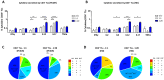
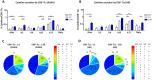
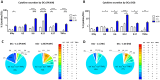
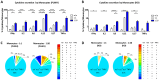
Introduction: The treatment of chronic viral infections can often bring viral replication under control. However, chronic immune activation persists and can lead to the development of comorbid conditions, such as cardiovascular disease and cancer. This is particularly true for people living with HIV (PLWH), who have significantly more extracellular vesicles from membrane budding, also called plasma microparticles (MPs), than healthy individuals (HDs), and a much more immunomodulatory phenotype. We hypothesized that the number and phenotypic heterogeneity of MPs can trigger a functional remodeling of immune responses in PLWH, preventing full immune restoration.
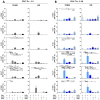
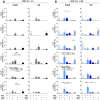
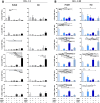
Methods: We investigated the rapid impact of three types of MPs - derived from membrane budding in platelets (CD41a+ PMPs), monocytes (CD14+ MMPs) and lymphocytes (CD3+ LMPs) in the plasma of PLWH or HDs-on four cell types (CD4+ and CD8+T lymphocytes, monocytes and DCs).
Results: These investigations of the short multiple interactions and functions of MPs with these cells revealed an increase in the secretion of cytokines such as IFNg, IL2, IL6, IL12, IL17 and TNFa by the immune cells studied following interactions with MPs. We show that this functional remodeling of immune cells depends not only on the number, but also on the phenotype of MPs.
Conclusion: These data suggest that the large numbers of MPs and their impact on functional remodeling in PLWH may be incompatible with the effective control of chronic infections, potentially leading to chronic immune activation and the onset of comorbid diseases.
Keywords: PLWH; cellular remodeling; cytokine secretion; extracellular vesicles (EV); immunomodulation.
Copyright © 2025 Neyrinck-Leglantier, Tamagne, Ben Rayana, Many, Pinheiro, Delorme, Andrieu, Boilard, Cognasse, Hamzeh-Cognasse, Perez-Patrigeon, Lelievre, Pirenne, Gallien and Vingert.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Immunoregulatory molecule expression on extracellular microvesicles in people living with HIV.Front Immunol. 2024 Mar 4;15:1354065. doi: 10.3389/fimmu.2024.1354065. eCollection 2024. Front Immunol. 2024. PMID: 38500878 Free PMC article.
-
Interactions with and activation of immune cells by CD41a+ extracellular vesicles.Front Immunol. 2025 Feb 14;16:1509078. doi: 10.3389/fimmu.2025.1509078. eCollection 2025. Front Immunol. 2025. PMID: 40028321 Free PMC article.
-
Potential Involvement of Platelet-Derived Microparticles and Microparticles Forming Immune Complexes during Monocyte Activation in Patients with Systemic Lupus Erythematosus.Front Immunol. 2018 Mar 1;9:322. doi: 10.3389/fimmu.2018.00322. eCollection 2018. Front Immunol. 2018. PMID: 29545790 Free PMC article. Clinical Trial.
-
Immune Cell-Derived Extracellular Vesicles - Functions and Therapeutic Applications.Trends Mol Med. 2019 May;25(5):382-394. doi: 10.1016/j.molmed.2019.02.003. Epub 2019 Mar 7. Trends Mol Med. 2019. PMID: 30853173 Review.
-
Tumor-derived microparticles in tumor immunology and immunotherapy.Eur J Immunol. 2020 Nov;50(11):1653-1662. doi: 10.1002/eji.202048548. Epub 2020 Oct 28. Eur J Immunol. 2020. PMID: 32976623 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

