Neutrophils negatively control IL-17A-producing γδ T cell frequencies in a contact-dependent manner under physiological conditions
- PMID: 40181985
- PMCID: PMC11965643
- DOI: 10.3389/fimmu.2025.1542191
Neutrophils negatively control IL-17A-producing γδ T cell frequencies in a contact-dependent manner under physiological conditions
Abstract
Background: In addition to serving as the primary effector cells against infections, neutrophils have been implicated in the regulation of both innate and adaptive immunity. In this study, we aimed to investigate the role of neutrophils in the regulation of the immune system under physiological conditions.
Methods: The in vivo effect of neutrophils on the immune system was examined using neutropenic mice. The interaction between neutrophils and γδ T cells was investigated using an in vitro co-culture system.
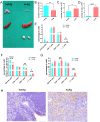
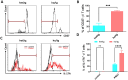
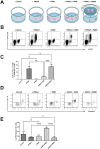
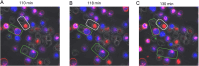
Findings: Unexpectedly, we observed an accumulation of γδ T cells in the cervical lymph nodes of neutropenic mice. Transcriptomic analysis revealed that these γδ T cells exhibited unique expression profiles of cell surface molecules and genes involved in defense responses. Further characterization indicated that the accumulated γδ T cells were IL-17 producing CD44+CD62L-CD27- memory cells. Additionally, in vitro experiments demonstrated that neutrophils could inhibit the function of IL-17A producing γδ T cells by inducing cell death in a contact-dependent manner.
Conclusion: This present study demonstrates that neutrophils negatively regulate IL-17 producing γδ T cells under physiological conditions. Given that IL-17A is a critical cytokine for the recruitment of neutrophils to peripheral tissues, our study suggests that the crosstalk between neutrophils and IL-17A producing γδ T cells is a crucial mechanism for maintaining immune homeostasis under physiological conditions.
Keywords: IL-17A; cell death; innate immunity; neutrophils; physiological condition; γδ T cells.
Copyright © 2025 Yu, Yue, Tchudjin Magatsin, Marwitz, Behrends, Goldmann, Opferman, Kasper and Petersen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

