Statement concerning the review of the approval of the basic substances chitosan and chitosan hydrochloride when used in plant protection
- PMID: 40182011
- PMCID: PMC11966830
- DOI: 10.2903/j.efsa.2025.9318
Statement concerning the review of the approval of the basic substances chitosan and chitosan hydrochloride when used in plant protection
Abstract
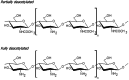
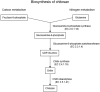
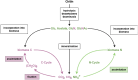
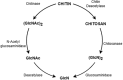
The European Commission asked EFSA to provide an opinion according to Article 23(6) of Regulation (EC) No 1107/2009, in conjunction with Article 29 of Regulation (EC) No 178/2002, regarding the approved plant protection uses of chitosan and chitosan hydrochloride as basic substances. The Panel on Plant Protection Products and their Residues (PPR) was not provided with new dossiers but collated available scientific and technical knowledge and used a weight of evidence approach and experts' judgement for its appraisal. The statement has considered the possibility for extrapolation of the toxicological properties between chitosan and chitosan hydrochloride, and whether both substances can be expected to be of no toxicological concern; a comparison between the estimated levels of chitosan and chitosan hydrochloride resulting from the approved uses as basic substances and the level of chitosan expected to naturally occur in the environment. This last comparison served to verify whether the approved uses as basic substances might lead to an exceedance of the expected natural background levels in any of the environmental compartments (quantitative for the soil compartment and (semi)quantitative for the freshwater compartment); and accordingly, whether there was a need to advise on the safety of chitosan and chitosan hydrochloride to non-target species occurring in the impacted environmental compartments. Overall, the PPR Panel concluded that toxicological properties can be extrapolated between chitosan and chitosan hydrochloride and that no toxicological concerns were identified. The estimated levels of chitosan and chitosan hydrochloride in the environment following application in accordance with their approved uses as basic substances would be within the same range, or below, the expected natural background exposure levels in soil and freshwaters. Considering the available ecotoxicological data and the environmental fate assessment, further consideration in relation to the safety to non-target organisms was considered not necessary. Missing information alongside related uncertainties have been identified and considered in the overall weight of the evidence.
Keywords: basic substances; chitin; chitosan; chitosan hydrochloride; environmental fate; mammalian toxicology; natural waters; pesticide; soil.
© 2025 European Food Safety Authority. EFSA Journal published by Wiley‐VCH GmbH on behalf of European Food Safety Authority.
Figures
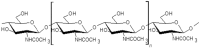
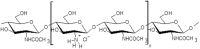
Similar articles
-
Chitin estimation in agricultural soils.EFSA J. 2025 Apr 3;23(4):e9313. doi: 10.2903/j.efsa.2025.9313. eCollection 2025 Apr. EFSA J. 2025. PMID: 40182010 Free PMC article.
-
Statement on the toxicological properties and maximum residue levels of acetamiprid and its metabolites.EFSA J. 2024 May 15;22(5):e8759. doi: 10.2903/j.efsa.2024.8759. eCollection 2024 May. EFSA J. 2024. PMID: 38751503 Free PMC article.
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
-
Scientific opinion on pesticides in foods for infants and young children.EFSA J. 2018 Jun 28;16(6):e05286. doi: 10.2903/j.efsa.2018.5286. eCollection 2018 Jun. EFSA J. 2018. PMID: 32625927 Free PMC article.
-
Basic Substances, a Sustainable Tool to Complement and Eventually Replace Synthetic Pesticides in the Management of Pre and Postharvest Diseases: Reviewed Instructions for Users.Molecules. 2022 May 28;27(11):3484. doi: 10.3390/molecules27113484. Molecules. 2022. PMID: 35684422 Free PMC article. Review.
Cited by
-
Chitin estimation in agricultural soils.EFSA J. 2025 Apr 3;23(4):e9313. doi: 10.2903/j.efsa.2025.9313. eCollection 2025 Apr. EFSA J. 2025. PMID: 40182010 Free PMC article.
References
-
- Abdel‐Ghany, H. M. , & Salem, M. E.‐S. (2020). Effects of dietary chitosan supplementation on farmed fish; a review. Reviews in Aquaculture, 12, 438–452. 10.1111/raq.12326 - DOI
-
- Abo Elsoud, M. M. , & El Kady, E. M. (2019). Current trends in fungal biosynthesis of chitin and chitosan. Bulletin of the National Research Centre, 43, 59. 10.1186/s42269-019-0105-y - DOI
-
- Ahmed, F. , Soliman, F. M. , Adly, M. A. , Soliman, H. A. M. , El‐Matbouli, M. , & Saleh, M. (2021). Dietary chitosan nanoparticles: Potential role in modulation of rainbow trout (Oncorhynchus mykiss) antibacterial defense and intestinal immunity against enteric Redmouth disease. Marine Drugs, 19, 72. 10.3390/md19020072 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
