Chemokine Ligands and Receptors Regulate Macrophage Polarization in Atherosclerosis: A Comprehensive Database Mining Study
- PMID: 40182401
- PMCID: PMC11963153
- DOI: 10.1016/j.cjco.2024.11.018
Chemokine Ligands and Receptors Regulate Macrophage Polarization in Atherosclerosis: A Comprehensive Database Mining Study
Abstract
Background: Atherosclerosis is a systemic disease involving multiple blood vessels and a major cause of cardiovascular disease. Current treatment methods (eg, statins) for atherosclerosis can reduce the risk of cardiovascular diseases effectively, but they are insufficient to completely reverse existing atherosclerosis. Macrophages play a central role in development of atherosclerosis. Chemokines, the main mediators of macrophage chemotaxis, are important in immune and inflammatory responses. The effects of chemokines on mechanisms involved in atherosclerosis are unknown. This study preliminarily investigated these effects and mechanisms via bioinformatics methods.
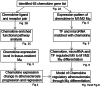
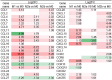
Methods: In this study, data on chemokine ligands and receptors were obtained by mining public databases (the National Center of Biotechnology Information-Gene Expression Omnibus [NCBI-GEO] database, ArrayExpress database, and single-cell RNA sequencing [scRNA-seq] database), and an extensive literature search was performed. The expression levels of chemokines in mouse tissues were analyzed via Metascape software for signalling pathway enrichment, scRNA-seq data for chemokine expression in atherosclerotic plaque progression and regression, and GEO2R data for chemokine expression during macrophage polarization. Ingenuity Pathway Analysis (IPA) software was used to analyze regulatory factors such as transcription factors and microRNAs that are significantly differentially expressed upstream of chemokines in macrophage polarization. Finally, a model of the chemokine regulation of atherosclerosis was established on the basis of these results.
Results: There are 5 main findings: (1) In atherosclerosis, chemokines are regulated by transcription factors and microRNAs. (2) The transcription factor STAT1 promotes the polarization of dormant (M0) macrophages into classically activated (M1) macrophages and alternative activated (M2) macrophages by regulating chemokines. The transcription factors STAT1, IRF7 and IRF1 regulate the polarization of M0 macrophages into M2a and M2b macrophages via different chemokines. For example, some transcription factors promote M1 polarization of M0 macrophages through CCL4, but M2 macrophage polarization is regulated via CCL19, CCL5 and CCR7. (3) Transcription factors can promote and inhibit, whereas miRNAs can only inhibit atherosclerosis. (4) CCL4 existed in all 5 different chemokine-regulated macrophage models, whereas CXCL3 only existed in the M2b macrophage transcriptional regulation model, indicating that CXCL3 may promote the M2b type macrophages polarization of M0 macrophages. (5) CCL5 and CCR7 can promote the M2a macrophages and M2b macrophages polarization of M0 macrophages.
Conclusions: Atherosclerosis can be treated by regulating chemokines and regulating the polarization of macrophages. The chemokines CCL4, CCL5, CCL8, CCL19, CXCL3, CXCL10, CXCL13, and CCR7 may play key roles in the progression and regression of atherosclerosis.
Contexte: L’athérosclérose, une affection généralisée qui touche de nombreux vaisseaux sanguins, est une des principales causes de maladies cardiovasculaires. Les traitements actuels (p. ex. les statines) permettent de réduire efficacement le risque de maladie cardiovasculaire, mais ils ne permettent pas à eux seuls de renverser complètement l’athérosclérose. Les macrophages jouent un rôle central dans l’apparition de l’athérosclérose. Les chimiokines, principaux médiateurs du chimiotactisme des macrophages, jouent un rôle important dans les réponses immunitaires et inflammatoires. Les effets des chimiokines sur les mécanismes qui interviennent dans l’athérosclérose ne sont pas connus. La présente étude préliminaire a examiné ces effets et mécanismes par des méthodes bio-informatiques.
Méthodologie: Dans cette étude, les données relatives aux ligands et aux récepteurs des chimiokines ont été obtenues par une recherche effectuée dans des bases de données publiques (National Center of Biotechnology Information-Gene Expression Omnibus [NCBI-GEO], ArrayExpress et une base de données scRNA-seq de transcriptomes de cellules uniques) de même que dans le cadre d’une revue exhaustive de la littérature médicale. Les chercheurs ont analysé le degré d’expression des chimiokines dans le tissu murin à l’aide ArrayExpress de Metascape pour l’enrichissement des voies de signalisation, et procédé à l’analyse du scRNA-seq pour mesurer l’expression des chimiokines dans la progression et la régression de la plaque athéromateuse; les données GEO2R ont permis de mesurer l’expression des chimiokines durant la polarisation macrophagique. Le logiciel Ingenuity Pathway Analysis (IPA) a servi à analyser les facteurs de régulation, comme les facteurs de transcription et les microARN, dont l’expression varie de façon marquée en amont de la polarisation des macrophages par les chimiokines. Finalement, un modèle de régulation des chimiokines dans l’athérosclérose a été mis au point à partir de ces résultats.
Résultats: Cinq constatations principales ont été dégagées : 1) Dans l’athérosclérose, la régulation des chimiokines repose sur les facteurs de transcription et les microARN. 2) Le facteur de transcription STAT1 favorise la polarisation des macrophages en dormance (M0) en phénotype M1 (macrophages activés par la voie classique) et en phénotype M2 (macrophages activés par d’autres voies) par les chimiokines régulatrices. Les facteurs de transcription STAT1, IRF7 et IRF1 régulent la polarisation des macrophages M0 en phénotypes M2a et M2b par différentes chimiokines. Par exemple, certains facteurs de transcription favorisent la polarisation des macrophages M0 en phénotype M1 par le CCL4, mais la polarisation des macrophages M2 est régulée par CCL19, CCL5 et CCR7. 3) Les facteurs de transcription peuvent favoriser ou inhiber l’athérosclérose, alors que les microARN peuvent seulement inhiber la progression de l’athérosclérose. 4) Le CCL4 était présent dans les cinq différents modèles de macrophages régulés par les chimiokines, alors que le CXCL3 était présent uniquement dans le modèle de régulation transcriptionnelle des macrophages M2b, ce qui indique que CXCL3 pourrait favoriser la polarisation des macrophages M0 en phénotype M2b. 5) Les CCL5 et CCR7 peuvent favoriser la polarisation des macrophages M0 en phénotypes M2a et M2b.
Conclusions: La régulation des chimiokines et la régulation de la polarisation des macrophages peuvent permettre de traiter l’athérosclérose. Les chimiokines CCL4, CCL5, CCL8, CCL19, CXCL3, CXCL10, CXCL13 et CCR7 pourraient jouer un rôle clé dans la progression et dans la régression de l’athérosclérose.
© 2024 The Authors.
Figures






Similar articles
-
Irf1- and Egr1-activated transcription plays a key role in macrophage polarization: A multiomics sequencing study with partial validation.Int Immunopharmacol. 2021 Oct;99:108072. doi: 10.1016/j.intimp.2021.108072. Epub 2021 Aug 16. Int Immunopharmacol. 2021. PMID: 34426111
-
The chemotaxis of M1 and M2 macrophages is regulated by different chemokines.J Leukoc Biol. 2015 Jan;97(1):61-9. doi: 10.1189/jlb.1A0314-170R. Epub 2014 Oct 30. J Leukoc Biol. 2015. PMID: 25359998
-
miR-130b-3p regulates M1 macrophage polarization via targeting IRF1.J Cell Physiol. 2021 Mar;236(3):2008-2022. doi: 10.1002/jcp.29987. Epub 2020 Aug 27. J Cell Physiol. 2021. PMID: 32853398
-
M2b macrophage polarization and its roles in diseases.J Leukoc Biol. 2019 Aug;106(2):345-358. doi: 10.1002/JLB.3RU1018-378RR. Epub 2018 Dec 21. J Leukoc Biol. 2019. PMID: 30576000 Free PMC article. Review.
-
Macrophage phenotypes in atherosclerosis.Immunol Rev. 2014 Nov;262(1):153-66. doi: 10.1111/imr.12218. Immunol Rev. 2014. PMID: 25319333 Review.
References
-
- Narula N., Olin J.W., Narula N. Pathologic disparities between peripheral artery disease and coronary artery disease. Arterioscler Thromb Vasc Biol. 2020;40:1982–1989. - PubMed
-
- Tabas I., Williams K.J., Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. - PubMed
-
- Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

