Advancements of paper-based microfluidics and organ-on-a-chip models in cosmetics hazards
- PMID: 40182506
- PMCID: PMC11966604
- DOI: 10.1039/d4ra07336c
Advancements of paper-based microfluidics and organ-on-a-chip models in cosmetics hazards
Abstract
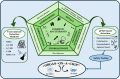
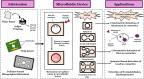
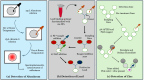
Cosmetics have been used in society for centuries for beautification and personal hygiene maintenance. Modern cosmetics include various makeup, hair, and skincare products that range from moisturizers and shampoos to lipsticks and foundations and have become a quintessential part of our daily grooming activities. However, dangerous adulterants are added during the production of these cosmetics, which range from heavy metals to microbial contaminants. These adulterants not only reduce the quality and efficacy of cosmetic products but also pose a significant risk to human health. Detecting the presence of adulterants in cosmetics is crucial for regulating substandard cosmetic products in the industry. The conventional methods to detect such adulterants and quality testing are expensive and take a lot of effort, particularly when involving advanced analytical detection and clinical trials. Recently, efficient methods such as microfluidic methods have emerged to detect adulterants rapidly. In this review, we mainly focus on various adulterants present in cosmetics and their detection using paper-based microfluidic devices. In addition, this review also sheds light on the organ-on-a-chip model with the goal of developing a human tissue model for cosmetic testing. Combined, these approaches provide an efficient, inexpensive, and sustainable approach for quality testing in the cosmetics industry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

