The role of public health in rare diseases: hemophilia as an example
- PMID: 40182514
- PMCID: PMC11965367
- DOI: 10.3389/fpubh.2025.1450625
The role of public health in rare diseases: hemophilia as an example
Abstract
Introduction: The role of public health has evolved from addressing infectious diseases to encompass non-communicable diseases. Individuals with genetic disorders and rare diseases constitute a particularly vulnerable population, requiring tailored public health policies, practical implementation strategies, and a long-term vision to ensure sustainable support. Given the prolonged duration and significant costs often associated with these conditions, comprehensive, patient-centered, and cost-effective approaches are essential to safeguard their physical and mental well-being.
Aims: To summarize definitions and concepts related to health, public health, rare diseases, and to highlight the role of integrating public health interventions into routine care in improving patient outcomes. Hemophilia was selected as an exemplary rare disease due to its significant lifetime treatment costs and the recent approval and pricing of its gene therapy as the world's most expensive drug, highlighting the critical importance of public health policies in ensuring equitable access to care and treatment.
Methods: A narrative literature review was conducted between July 2023 and December 2024, searching PubMed, Google Scholar, and Google for various topics related to rare diseases, public health, and hemophilia.
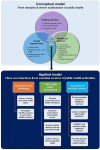
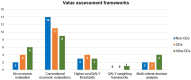
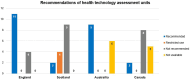
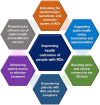
Results: Public health can play an important role in improving the health outcomes of people with rare diseases by implementing conceptual and applied models to accomplish a set of objectives. Over the past two decades, legislative and regulatory support in high income countries (HICs) has facilitated the development and approval of diagnostics and treatments for several rare diseases leading to important advancements. In contrast, many low- and middle-income countries (LMICs) face obstacles in enacting legislation, developing regulations, and implementing policies to support rare disease diagnosis and treatment. More investment and innovation in drug discovery and market access pathways are still needed in both LMICs and HICs. Ensuring the translation of public health policies into regulatory measures, and in turn implementing, and regularly evaluating these measures to assess their effectiveness is crucial. In the case of hemophilia, public health can play a pivotal role.
Conclusion: Enhancing public health surveillance, policies, and interventions in hemophilia and other rare diseases can bridge data gaps, support access to equitable treatment, promote evidence-based care, and improve outcomes across the socioeconomic spectrum.
Keywords: disease burden; health inequities; hemophilia; orphan drugs; public health; public health policy; public health surveillance; rare diseases.
Copyright © 2025 El-Sayed, Reiss, Hanna and Bolous.
Conflict of interest statement
AE-S is an employee at Novo Nordisk Egypt. DH is an employee at Phoenix Clinical Research. The conception, design, and conduct of this research project were done completely independently of their employers. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Dale D, Groft S, Harrison M. Rare disease registries In: Gliklich RE, Leavy MB, Dreyer NA, editors. Registries for evaluating patient outcomes: a user’s guide, vol. 2. 3rd ed: Agency for Healthcare Research and Quality (US) (2014). 113–34. Available at: https://effectivehealthcare.ahrq.gov/sites/default/files/wysiwyg/registr... - PubMed
-
- Abuhadida S, Bastaki L, Bash B, Alhindal B. Return on investment from the prevention of orphan diseases in Kuwait. Ann Public Heal. (2022) 1:637. doi: 10.55085/aph.2022.637 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

