Development of a Predictive Model for Classifying Immune Checkpoint Inhibitor-Induced Liver Injury Types
- PMID: 40182662
- PMCID: PMC11966236
- DOI: 10.1002/jgh3.70147
Development of a Predictive Model for Classifying Immune Checkpoint Inhibitor-Induced Liver Injury Types
Abstract
Aims: Immune checkpoint inhibitors (ICIs) have transformed cancer therapy; however, they are associated with ICI-induced liver injury (ICI-LI), which manifests as hepatocellular, mixed, or cholestatic patterns with variable treatment responses. This study aimed to develop and validate a predictive model to identify ICI-LI type using clinical data available at ICI initiation.
Methods: A retrospective analysis of 297 patients with ICI-LI was conducted. Baseline clinical data were analyzed using univariate and multivariate logistic regression to predict ICI-LI types in the training and validation cohorts. A predictive model was developed and validated using receiver operating characteristic (ROC) curve analysis.
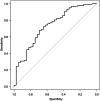
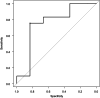
Results: Multivariate analysis in the training cohort identified male sex (odds ratio [OR]: 3.33, 95% confidence interval [CI]: 1.57-7.06, p = 0.002), serum albumin levels (OR: 0.42, 95% CI: 0.19-0.91, p = 0.027), and serum alanine aminotransferase (ALT) levels (OR: 0.97, 95% CI: 0.94-0.99, p = 0.015) as significant predictors, along with ICI regimen types selected using the Akaike information criterion. The logistic regression model, expressed as p = 1/{1 + (-(5.02 + 1.20 × (sex [F:0, M:1])) - 0.87 × albumin [g/dL] - 0.03 × ALT [U/L] - 0.9 × (drug [non-anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) related regimen:0, anti-CTLA-4 related regimen:1]))}, achieved an area under the ROC (AUROC) of 0.73 (95% CI: 0.63-0.82) in the training cohort. At a cut-off of 0.86, the sensitivity was 60.3%, specificity 74.4%, positive predictive value 92.3%, and negative predictive value 26.9%. In the validation cohort, the AUROC was 0.752 (95% CI: 0.476-1.00).
Conclusion: This predictive model demonstrates its utility in classifying ICI-LI types.
Keywords: immune checkpoint inhibitors; immune checkpoint inhibitor‐induced liver injury; immune‐related adverse events; predictive model; treatment management.
© 2025 The Author(s). JGH Open published by Journal of Gastroenterology and Hepatology Foundation and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Salman M., Varun S., Julie D., and Eyad E., “Immune Checkpoints in the Tumor Environment,” Seminars in Cancer Biology 65 (2020): 1–12. - PubMed
-
- Kubo T., Sugawara T., Shinkawa T., et al., “Fatal Fulminant Hepatitis Induced by Combined Ipilimumab and Nivolumab Therapy Despite Favorable Histologic Response and Confirmed by Autopsy in a Patient With Clear Cell Renal Carcinoma,” Immunological Medicine 44 (2021): 136–141. - PubMed
-
- Cunningham M., Gupta R., and Butler M., “Checkpoint Inhibitor Hepatotoxicity: Pathogenesis and Management,” Hepatology 79 (2024): 198–212. - PubMed
LinkOut - more resources
Full Text Sources

