Towards propagation of epidermal cells for wound repair: glass, as cell culture substrate, enhances proliferation and migration of human keratinocytes
- PMID: 40182989
- PMCID: PMC11965597
- DOI: 10.3389/fbioe.2025.1547044
Towards propagation of epidermal cells for wound repair: glass, as cell culture substrate, enhances proliferation and migration of human keratinocytes
Abstract
Introduction: Human keratinocytes require relatively long propagation time which impedes their availability as autologous cell transplantation within a clinically reasonable timeframe. There is an unmet need for efficient xeno-free cell expansion approaches to propagate human keratinocytes as regenerative therapy.
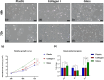
Methods: Primary human keratinocytes and HaCaT cells were cultured on glass, plastic, and animal-derived collagen I matrix for 10 days. Proliferation, migration, DNA methylation, as well as gene and protein expression were assessed to characterize the effect of the tested culture substrates on keratinocytes at the molecular and functional levels.
Results: Keratinocytes cultured on glass exhibited faster proliferation, global DNA demethylation and upregulation of epidermal differentiation markers. Scratch wound assay revealed that keratinocytes cultured on glass demonstrated enhanced cell migration compared to those on plastic or collagen I. Multiplex immunoassays identified temporal and substrate-dependent variations in a panel of keratinocyte-specific secreted factors, encompassing immunomodulatory cytokines, growth factors, and angiogenic factors.
Discussion: Glass, as a culture substrate, promotes epidermal differentiation and enhances keratinocyte migration. The latter is a critical factor in re-epithelialization and wound healing. Functional properties suggest that glass may optimize the inflammatory response and promote efficient wound repair, making it a promising candidate for the short-term expansion of keratinocytes for transplantation purposes. Further in-vivo validation is required to definitively establish the efficacy of keratinocytes cultured on glass for clinical applications.
Keywords: ATMP; culture substrate; epidermal differentiation; glass; keratinocytes; migration; wound healing.
Copyright © 2025 Shahin, Steinvall, Sjöberg, Elmasry and El-Serafi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Cathepsin B is essential for regeneration of scratch-wounded normal human epidermal keratinocytes.Eur J Cell Biol. 2007 Dec;86(11-12):747-61. doi: 10.1016/j.ejcb.2007.03.009. Epub 2007 Jul 24. Eur J Cell Biol. 2007. PMID: 17651862
-
Using paracrine effects of Ad-MSCs on keratinocyte cultivation and fabrication of epidermal sheets for improving clinical applications.Cell Tissue Bank. 2018 Dec;19(4):531-547. doi: 10.1007/s10561-018-9702-5. Epub 2018 Aug 13. Cell Tissue Bank. 2018. PMID: 30105667
-
Keratinocyte autophagy enables the activation of keratinocytes and fibroblastsand facilitates wound healing.Autophagy. 2021 Sep;17(9):2128-2143. doi: 10.1080/15548627.2020.1816342. Epub 2020 Sep 18. Autophagy. 2021. PMID: 32866426 Free PMC article.
-
Wound re-epithelialization: modulating keratinocyte migration in wound healing.Front Biosci. 2007 May 1;12:2849-68. doi: 10.2741/2277. Front Biosci. 2007. PMID: 17485264 Review.
-
Keratinocyte Growth Factor-Based Strategies for Wound Re-Epithelialization.Tissue Eng Part B Rev. 2022 Jun;28(3):665-676. doi: 10.1089/ten.TEB.2021.0030. Epub 2021 Oct 18. Tissue Eng Part B Rev. 2022. PMID: 34238035 Review.
References
-
- Alghfeli L., Parambath D., Manzoor S., Roach H. I., Oreffo R. O. C., El-Serafi A. T. (2021). Synthesis of scaffold-free, three dimensional, osteogenic constructs following culture of skeletal osteoprogenitor cells on glass surfaces. Bone Rep. 15, 101143. 10.1016/j.bonr.2021.101143 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

