Pharmacological evaluation of drug therapies in Aicardi-Goutières syndrome: insights from patient-derived neural stem cells
- PMID: 40183101
- PMCID: PMC11966042
- DOI: 10.3389/fphar.2025.1549183
Pharmacological evaluation of drug therapies in Aicardi-Goutières syndrome: insights from patient-derived neural stem cells
Abstract
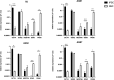
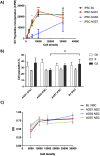
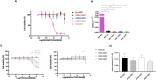
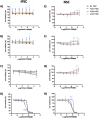
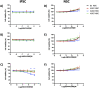
Aicardi-Goutières syndrome (AGS) is a rare genetic disorder classified among type I interferonopathies. Current pharmacological management of AGS is symptomatic and supportive, with recent clinical applications of JAK inhibitors (JAKi) and antiretroviral therapies (RTIs). To investigate the effects of these therapies, patient-specific induced pluripotent stem cells (iPSCs) were generated by reprogramming fibroblasts from three AGS patients with distinct genetic mutations (AGS1, AGS2, AGS7) and differentiated into neural stem cells (NSCs). iPSCs and NSCs derived from commercial BJ fibroblasts of a healthy donor served as control. The cytotoxic effects of glucocorticoids, thiopurines, JAK inhibitors (ruxolitinib, baricitinib, tofacitinib, pacritinib), and RTIs (abacavir, lamivudine, zidovudine) were evaluated using the MTT assay. Results showed that glucocorticoids did not compromise NSC viability. Among thiopurines, thioguanine, but not mercaptopurine, exhibited cytotoxicity in NSCs. All tested JAK inhibitors, except pacritinib, were non-toxic to iPSCs and NSCs. Interestingly, high concentrations of certain JAK inhibitors (ruxolitinib, baricitinib, tofacitinib) led to an unexpected increase in cell viability in AGS patient-derived cells compared to control, suggesting potential alterations in cell proliferation or stress responses. RTIs demonstrated no cytotoxicity, except for zidovudine, which showed selective toxicity in AGS2-derived iPSCs compared to controls. These findings suggest that glucocorticoids, JAK inhibitors (excluding pacritinib), and RTIs are likely safe for NSCs of AGS patients, while caution is warranted with thioguanine and pacritinib. Further studies are needed to explore the mechanisms underlying increased cell viability at high JAK inhibitor concentrations and the selective sensitivity to zidovudine.
Keywords: Aicardi-Goutières syndrome; JAK inhibitors; antiretrovirals; drug sensitivity; patient-derived stem cell.
Copyright © 2025 Braidotti, Ferraro, Franca, Genova, Giambuzzi, Mancini, Marinozzi, Pugnetti, Zudeh, Tesser, Tommasini, Decorti, Giliani and Stocco.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Akwa Y., Hassett D. E., Eloranta M. L., Sandberg K., Masliah E., Powell H., et al. (1998). Transgenic expression of IFN-α in the central nervous system of mice Protects against Lethal Neurotropic viral Infection but Induces inflammation and Neurodegeneration. J. Immunol. 161 (9), 5016–5026. 10.4049/jimmunol.161.9.5016 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

