Bifidobacterium Lactobacillus Triple Viable Alleviates Slow Transit Constipation by Regulating Gut Microbiota and Metabolism
- PMID: 40183209
- PMCID: PMC12136808
- DOI: 10.1111/jgh.16960
Bifidobacterium Lactobacillus Triple Viable Alleviates Slow Transit Constipation by Regulating Gut Microbiota and Metabolism
Abstract
Background: Gut microbiota plays a crucial role in the pathogenesis and treatment of functional constipation (FC). The aim of this study was to explore the therapeutic effects of Bifidobacterium Lactobacillus triple viable on slow transit constipation (STC).
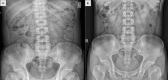
Methods: Patients with STC who met the Rome IV criteria received Bifidobacterium Lactobacillus triple viable. Gastrointestinal transit time (GITT) and constipation-related symptoms were assessed before and after receiving Bifidobacterium Lactobacillus triple viable. Additionally, a rat STC model was induced by loperamide and was treated with Bifidobacterium Lactobacillus triple viable to evaluate whether Bifidobacterium Lactobacillus triple viable could improve constipation in the rats and to explore the possible mechanisms involved.
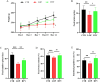
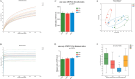
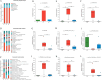
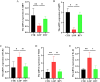
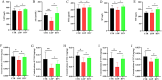
Results: In patients with STC, Bifidobacterium Lactobacillus triple viable accelerated GITT and improved constipation-related symptoms, including bowel movement frequency, hard bowel movement, incomplete defecation, defecation time, purgative measures, and stool form. In addition, Bifidobacterium Lactobacillus triple viable improved body weight, food intake, bowel movement, the fecal water content, and the intestinal propulsion rate in STC rats. It regulates the gut microbiota structure in rats; increases serum acetylcholine (Ach), 5-hydroxytryptamine (5-HT), substance P (SP), and vasoactive intestinal peptide (VIP); increases fecal long-chain fatty acids (LCFAs); upregulates the mRNA expression of aquaporin 3 (AQP3) and aquaporin 3 (AQP8); and downregulates the mRNA expression of Toll-like receptor 2 (TLR2), Toll-like receptor 4 (TLR4), and interleukin-1β (IL-1β).
Conclusions: Bifidobacterium Lactobacillus triple viable ameliorated the GITT and constipation-related symptoms of patients with STC and improved the STC in rats by regulating the gut microbiota and metabolism.
Keywords: Bifidobacterium Lactobacillus triple viable; gut microbiota; metabolism; slow transit constipation.
© 2025 The Author(s). Journal of Gastroenterology and Hepatology published by Journal of Gastroenterology and Hepatology Foundation and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Poly-γ-glutamic acid alleviates slow transit constipation by regulating aquaporin and gut microbes.Sci Rep. 2025 Mar 10;15(1):8244. doi: 10.1038/s41598-025-92783-2. Sci Rep. 2025. PMID: 40065004 Free PMC article.
-
Tongbian formula alleviates slow transit constipation by increasing intestinal butyric acid to activate the 5-HT signaling.Sci Rep. 2024 Aug 2;14(1):17951. doi: 10.1038/s41598-024-68473-w. Sci Rep. 2024. PMID: 39095450 Free PMC article.
-
Correlation between slow transit constipation and spleen deficiency, and gut microbiota: a pilot study.J Tradit Chin Med. 2022 Jun;42(3):353-363. doi: 10.19852/j.cnki.jtcm.20220408.002. J Tradit Chin Med. 2022. PMID: 35610004 Free PMC article.
-
Lactic acid bacteria in relieving constipation: mechanism, clinical application, challenge, and opportunity.Crit Rev Food Sci Nutr. 2025;65(3):551-574. doi: 10.1080/10408398.2023.2278155. Epub 2023 Nov 16. Crit Rev Food Sci Nutr. 2025. PMID: 37971876 Review.
-
Roles of Gut Microbiota and Metabolites in Pathogenesis of Functional Constipation.Evid Based Complement Alternat Med. 2021 Sep 21;2021:5560310. doi: 10.1155/2021/5560310. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34603471 Free PMC article. Review.
References
-
- Wang H. L., “Understanding the Pathogenesis of Slow‐Transit Constipation: One Step Forward,” Digestive Diseases and Sciences 60 (2015): 2216–2218. - PubMed
-
- Tomita R., Tanjoh K., Fujisaki S., Ikeda T., and Fukuzawa M., “Regulation of the Enteric Nervous System in the Colon of Patients With Slow Transit Constipation,” Hepato‐Gastroenterology 49 (2002): 1540–1544. - PubMed
-
- Björnsson E. S., Chey W. D., Hooper F., Woods M. L., Owyang C., and Hasler W. L., “Impaired Gastrocolonic Response and Peristaltic Reflex in Slow‐Transit Constipation: Role of 5‐HT(3) Pathways,” American Journal of Physiology. Gastrointestinal and Liver Physiology 283 (2002): G400–G407. - PubMed
-
- Boeckxstaens G., Camilleri M., Sifrim D., et al., “Fundamentals of Neurogastroenterology: Physiology/Motility ‐ Sensation,” Gastroenterology 150 (2016): 1292–1304.e2. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

