Population-level impact of semaglutide 2.4 mg in patients with obesity or overweight and cardiovascular disease: A modelling study based on the SELECT trial
- PMID: 40183412
- PMCID: PMC12046440
- DOI: 10.1111/dom.16370
Population-level impact of semaglutide 2.4 mg in patients with obesity or overweight and cardiovascular disease: A modelling study based on the SELECT trial
Abstract
Aim: To estimate the impact of semaglutide 2.4 mg treatment on the risk of major adverse cardiovascular events (MACE) in adults with overweight/obesity in the United States based on the SELECT trial of patients with atherosclerotic cardiovascular disease.
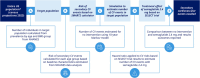
Materials and methods: Using 2023 census projections and National Health and Nutrition Examination Survey data, we developed Markov population-based predictive models for US adults meeting SELECT inclusion criteria and, separately, for adults eligible for semaglutide 2.4 mg for its MACE risk reduction indication. The 10-year rate of recurrent MACE and deaths was estimated based on the Secondary Manifestations of ARTerial disease 2 risk calculator and estimated semaglutide 2.4 mg treatment effect as per the SELECT MACE hazard ratio.
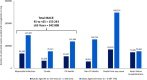
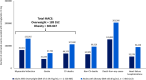
Results: Of 6 164 019 US adults meeting the SELECT criteria, 2 523 218 (40.9%) are estimated to have ≥1 new MACE in the next 10 years with no additional intervention. Semaglutide 2.4 mg may prevent 496 400 events, a 16% relative reduction. An estimated 2 103 630 deaths are predicted over the next 10 years, of which 332 597 deaths (any cause, 16% relative reduction) could be avoided with semaglutide 2.4 mg. Among the estimated 22 653 158 meeting the MACE risk reduction FDA label criteria, 42.7% could experience ≥1 new MACE; treatment could prevent 1 934 493 MACE and 1 231 295 deaths (16% relative reduction for both).
Conclusion: Four in 10 individuals in the United States meeting the SELECT criteria are estimated to experience a recurrent CV event without additional intervention. Semaglutide 2.4 mg can potentially prevent between half a million and up to 2 million MACE over the next 10 years in the population meeting SELECT and MACE risk reduction eligibility.
Plain language summary: What is the context and purpose of this research study? More than 7 in 10 US adults have overweight or obesity, which increases the risk of heart disease. Semaglutide is a medication used to treat type 2 diabetes and obesity. A clinical study called SELECT found that semaglutide reduces the risk of heart attack, stroke, or death by 20% in adults with overweight or obesity and heart disease. What was done? Our research estimated how many people in the United States would meet the criteria for participation in SELECT, how many heart disease events they might have with regular medical care over the next 10 years, and how many could be avoided with semaglutide 2.4 mg treatment in addition to regular medical care. We also estimated how many people would still be alive if they were treated with semaglutide. We estimated the same information for all people eligible for treatment with semaglutide based on the US Food and Drug Administration (FDA) indication of semaglutide 2.4 mg in patients with heart disease. These estimations were based on a large survey of US adults. What were the main results? We found that over 6 million people would meet the SELECT study criteria. Of these, 41% are estimated to have at least 1 new heart disease event in the next 10 years. If treated with semaglutide 2.4 mg, nearly 500 000 heart disease events and more than 300 000 deaths could be avoided. More than 22 million adults would qualify for semaglutide 2.4 mg, according to the FDA indication. If all of these people were treated with semaglutide 2.4 mg, nearly 2 million heart disease events and more than 1 million deaths might be prevented. What is the originality and relevance of this study? Treatment with semaglutide 2.4 mg can reduce the risk of new heart disease events and death in patients with existing heart disease, showing a substantial impact of semaglutide treatment in a real-world setting in the United States. Our study used different analyses to add to the existing research about reducing the risk of heart disease in people with overweight or obesity.
Keywords: United States; cardiovascular disease; cardiovascular risk; glucagon‐like peptide‐1 receptor agonists; obesity; overweight; semaglutide.
© 2025 The Author(s). Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
MGN is a consultant for Merck, Novo Nordisk and HeartFlow, Inc. He reports current research support from the American College of Cardiology Foundation supported by the George F. and Ann Harris Bellows Foundation, the Patient‐Centered Outcomes Research Institute and the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342). AMN reports funding for research to her institution from Esperion and Amgen, and honoraria and consulting fees from Amgen, Eli Lilly, Esperion, Janssen, Merck, NewAmsterdam, Novo Nordisk, Novartis, Pfizer and Silence Therapeutics. Her spouse receives research funding to his institution from Amgen and Esperion, and consulting from Janssen and Amgen. MF, JCT, AF, ASH are employees and shareholders of Novo Nordisk Inc. ADH, TSS and AL are employed by Genesis Research Group, a consulting company that received funding from Novo Nordisk to carry out this research. QVD was employed by Genesis Research Group at the time the research was conducted.
Figures
Similar articles
-
Semaglutide and cardiovascular outcomes in patients with obesity and prevalent heart failure: a prespecified analysis of the SELECT trial.Lancet. 2024 Aug 24;404(10454):773-786. doi: 10.1016/S0140-6736(24)01498-3. Lancet. 2024. PMID: 39181597 Clinical Trial.
-
Cardiovascular event reduction among a US population eligible for semaglutide per the SELECT trial.Am Heart J. 2024 Oct;276:110-114. doi: 10.1016/j.ahj.2024.05.007. Epub 2024 Jul 30. Am Heart J. 2024. PMID: 39182939
-
US Population Eligibility and Estimated Impact of Semaglutide Treatment on Obesity Prevalence and Cardiovascular Disease Events.Cardiovasc Drugs Ther. 2025 Feb;39(1):75-84. doi: 10.1007/s10557-023-07488-3. Epub 2023 Aug 14. Cardiovasc Drugs Ther. 2025. PMID: 37578663 Free PMC article.
-
[New outcome studies with injectable semaglutide in different at risk populations].Rev Med Liege. 2024 Oct;79(10):676-682. Rev Med Liege. 2024. PMID: 39397557 Review. French.
-
Long-acting GLP-1 receptor agonists: Findings and implications of cardiovascular outcomes trials.JAAPA. 2020 Aug;33(8):19-30. doi: 10.1097/01.JAA.0000669452.63883.45. JAAPA. 2020. PMID: 32740122 Review.
References
-
- National Institute of Diabetes and Digestive Kidney Diseases . Overweight & Obesity Statistics. National Institutes of Health. Accessed August 30, 2024. https://www.niddk.nih.gov/health-information/health-statistics/overweigh...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

