Deficiency of the Synaptic Adhesion Protein Leucine-Rich Repeat Transmembrane Protein 4 Like 1 Affects Anxiety and Aggression in Zebrafish
- PMID: 40183503
- PMCID: PMC11970230
- DOI: 10.1111/apha.70042
Deficiency of the Synaptic Adhesion Protein Leucine-Rich Repeat Transmembrane Protein 4 Like 1 Affects Anxiety and Aggression in Zebrafish
Abstract
Aim: Leucine-rich repeat transmembrane proteins (LRRTMs) are synaptic adhesion proteins that regulate synapse development and function. They interact transsynaptically with presynaptic binding partners to promote presynaptic differentiation. Polymorphisms of LRRTM4, one of the four members of this protein family, have been linked to multiple neuropsychiatric disorders and childhood aggression, but the underlying mechanisms and physiological function of LRRTM4 during behavior are currently unclear.
Methods: To characterize the role of this gene for brain function, we combined a battery of behavioral assays with transcriptomic and metabolomic analyses, using zebrafish as a model system.
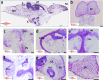
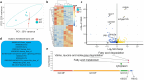
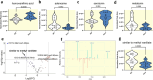
Results: Our findings revealed that lrrtm4l1, a brain-specific zebrafish orthologue of human LRRTM4, exhibits a brain region-specific expression pattern similar to humans, with strong expression in the dorsal telencephalon, a brain area critical for regulating emotional-affective and social behavior. lrrtm4l1-/- zebrafish displayed heightened anxiety and reduced aggression, while locomotion and social behavior remained unaffected by the gene knockout. Transcriptomic analysis of the telencephalon identified over 100 differentially expressed genes between wild-type and mutant zebrafish and an enrichment of pathways related to synaptic plasticity and neuronal signaling. The brain metabolome of lrrtm4l1-/- zebrafish showed multiple alterations, particularly in the dopaminergic and adenosinergic neurotransmitter systems.
Conclusion: These findings suggest that LRRTMs may have functions beyond their established role in excitatory synapse development, such as the regulation of neurotransmission and behavior. Targeting LRRTM4 therapeutically may thus be an interesting novel approach to alleviate excessive aggression or anxiety associated with a number of neuropsychiatric conditions.
Keywords: aggression; anxiety; leucine‐rich repeat transmembrane protein 4; neurotransmission; synaptic plasticity.
© 2025 The Author(s). Acta Physiologica published by John Wiley & Sons Ltd on behalf of Scandinavian Physiological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
A Neurexin2aa deficiency results in axon pathfinding defects and increased anxiety in zebrafish.Hum Mol Genet. 2021 Feb 4;29(23):3765-3780. doi: 10.1093/hmg/ddaa260. Hum Mol Genet. 2021. PMID: 33276371
-
LRRTMs Organize Synapses through Differential Engagement of Neurexin and PTPσ.Neuron. 2020 Apr 8;106(1):108-125.e12. doi: 10.1016/j.neuron.2020.01.003. Epub 2020 Jan 28. Neuron. 2020. PMID: 31995730
-
A Novel Function of the Lysophosphatidic Acid Receptor 3 (LPAR3) Gene in Zebrafish on Modulating Anxiety, Circadian Rhythm Locomotor Activity, and Short-Term Memory.Int J Mol Sci. 2020 Apr 18;21(8):2837. doi: 10.3390/ijms21082837. Int J Mol Sci. 2020. PMID: 32325720 Free PMC article.
-
Role of LRRTMs in synapse development and plasticity.Neurosci Res. 2017 Mar;116:18-28. doi: 10.1016/j.neures.2016.10.003. Epub 2016 Oct 31. Neurosci Res. 2017. PMID: 27810425 Review.
-
The leucine-rich repeat superfamily of synaptic adhesion molecules: LRRTMs and Slitrks.Mol Cells. 2012 Oct;34(4):335-40. doi: 10.1007/s10059-012-0113-3. Epub 2012 Jul 4. Mol Cells. 2012. PMID: 22767246 Free PMC article. Review.
References
-
- Laurén J., Airaksinen M. S., Saarma M., and Timmusk T., “A Novel Gene Family Encoding Leucine‐Rich Repeat Transmembrane Proteins Differentially Expressed in the Nervous System,” Genomics 81, no. 4 (2003): 411–421. - PubMed
-
- Song Y. S. and Kim E., “Presynaptic Proteoglycans: Sweet Organizers of Synapse Development,” Neuron 79, no. 4 (2013): 609–611. - PubMed
-
- Siddiqui T. J., Tari P. K., Connor S. A., et al., “An LRRTM4‐HSPG Complex Mediates Excitatory Synapse Development on Dentate Gyrus Granule Cells,” Neuron 79, no. 4 (2013): 680–695. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

