AOC 1044 induces exon 44 skipping and restores dystrophin protein in preclinical models of Duchenne muscular dystrophy
- PMID: 40183632
- PMCID: PMC11969676
- DOI: 10.1093/nar/gkaf241
AOC 1044 induces exon 44 skipping and restores dystrophin protein in preclinical models of Duchenne muscular dystrophy
Abstract
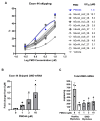
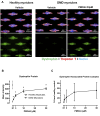
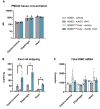
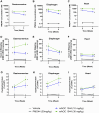
Duchenne muscular dystrophy (DMD) is a severe disorder caused by mutations in the dystrophin gene, resulting in loss of functional dystrophin protein in muscle. While phosphorodiamidate morpholino oligomers (PMOs) are promising exon-skipping therapeutics aimed at restoring dystrophin expression, their effectiveness is often limited by poor muscle delivery. We developed AOC 1044, an antibody-oligonucleotide conjugate (AOC) that combines a PMO-targeting exon 44 with an antibody against the transferrin receptor (TfR1), enhancing delivery to muscle tissues for patients with DMD amenable to exon 44 skipping (DMD44). AOC 1044 induces dose-dependent exon 44 skipping and its mouse-active variant elicited dose-dependent dystrophin restoration in skeletal and cardiac muscle in a DMD mouse model. This treatment also reduced muscle damage, as evidenced by decreases in serum creatine kinase and key liver enzymes, suggesting that restored dystrophin is functionally active. In nonhuman primates, single or repeated AOC 1044 doses resulted in dose-dependent increases in PMO concentration and exon 44 skipping across a range of muscle tissues, including the heart. Collectively, these findings highlight AOC 1044 as a promising therapeutic candidate for patients with DMD44, offering improved muscle targeting and meaningful dystrophin restoration, with potential clinical benefits in reducing muscle degeneration.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
Usue Etxaniz, Isaac Marks, Michael Cochran, and Venkata R. Doppalapudi are employees of Avidity Biosciences, Inc. who receive stock and stock options and have patent applications or pending or awarded patents. Matthew Diaz, Aaron Anderson, Olecya Tyaglo, Tiffany Hoang, Maria Azzurra Missinato, Kristoffer Svensson, Ben Badillo, Philip R. Kovach, Laura Leung, Hae Won Kwon, Husam S. Younis, W. Michael Flanagan, Arthur A. Levin, Hanhua Huang, and Georgios Karamanlidis are employees of Avidity Biosciences, Inc. who receives stock and stock options. Tyler Albin, Raghav Bhardwaj, Maria Azzurra Missinato, and Beatrice Darimont are former employees of Avidity Biosciences, Inc and may hold stock and/or stock options (and Tyler Albin and Beatrice Darimont have patent applications). Toshifumi Yokota and Rika Maruyama are co-founders and shareholders of OligomicsTX, Inc.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

